
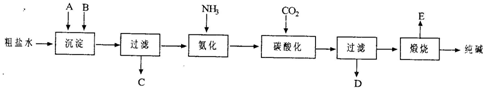
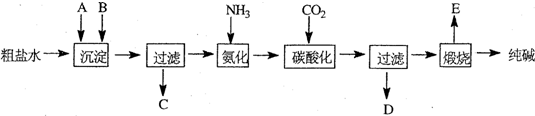
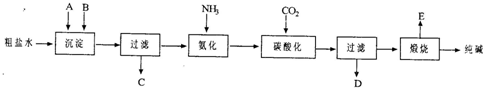
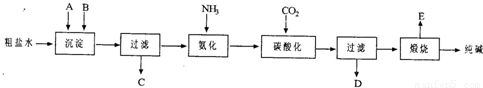
��20�֣���ҵ��������Ĺ�������ʾ��ͼ���£�
���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������д��A��B�Ļ�ѧʽA ____________________ B _______________________
��2��ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����_______ ��������_______ ��_______ ����ȴ�ᾧ��_______ �����
��3����ҵ��������������У�̼�ữʱ������������__________________________
̼�ữʱû������̼���ƾ��壬��ԭ����____________________________________
��4��̼�ữ����ˣ���ҺD����Ҫ�ijɷ���__________________________����д��ѧʽ����������һ�ɷֵ������ӵľ��巽���ǣ�___________________________________
��5����������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��__________________________________________��ҺD��ʯ��ˮǰ��Ҫ���ȣ�ԭ����___________________________________________
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��____________________________
��ע����ı���ʽ�����õ��йط��ŵĺ��壩

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| 84(m1-m2) |
| 31m1 |
| 84(m1-m2) |
| 31m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ͽ�����ģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��������Ͽ����߿���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com