
��ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A����aL��V��B����0��8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g����3H2��g�� 2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��
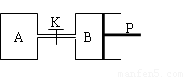
��B��N2��ת������ ��A��N2��ת���� ������ڡ��������ڡ���С�ڡ���B��N2��ת���ʡ������� ��
�ƴ�K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ ����˵�����ɣ�
��A��Ӧ�ﵽƽ��ʱ���ų�akJ������������A�г���2molNH3������ͬ�����´ﵽƽ��ʱ��������ΪbkJ����a��b�Ĺ�ϵ�� ��
��B�з�Ӧ�ﵽƽ���ֻ�ı�����������c��NH3�����ı���� ������ţ���
A�������¶� B������������1molAr��g��
C���������г���2molNH3 D��ѡ��Ч�����õĴ���
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A��=aL��V��B��=0.8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g��+3H2��g��
ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A��=aL��V��B��=0.8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g��+3H2��g��  2NH3��g������H=-92.4kJ/mol���ﵽƽ���V��B��=0.6aL��
2NH3��g������H=-92.4kJ/mol���ﵽƽ���V��B��=0.6aL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A����aL��V��B����0��8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g����3H2��g�� ![]() 2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��
2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��
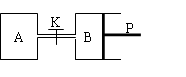
��B��N2��ת������ ��A��N2��ת���� ������ڡ��������ڡ���С�ڡ���B��N2��ת���ʡ������� ��
�ƴ�K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ ����˵�����ɣ�
��A��Ӧ�ﵽƽ��ʱ���ų�akJ������������A�г���2molNH3������ͬ�����´ﵽƽ��ʱ��������ΪbkJ����a��b�Ĺ�ϵ�� ��
��B�з�Ӧ�ﵽƽ���ֻ�ı�����������c��NH3�����ı���� ������ţ���
A�������¶� B������������1molAr��g��
C���������г���2molNH3 D��ѡ��Ч�����õĴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A����aL��V��B����0��8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g����3H2��g�� 2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��
��B��N2��ת������ ��A��N2��ת���� ������ڡ��������ڡ���С�ڡ���B��N2��ת���ʡ������� ��
�ƴ�K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ ����˵�����ɣ�
��A��Ӧ�ﵽƽ��ʱ���ų�akJ������������A�г���2molNH3������ͬ�����´ﵽƽ��ʱ��������ΪbkJ����a��b�Ĺ�ϵ�� ��
��B�з�Ӧ�ﵽƽ���ֻ�ı�����������c��NH3�����ı���� ������ţ���
A�������¶� B������������1molAr��g��
C���������г���2molNH3 D��ѡ��Ч�����õĴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ������ѧ������һ�и�����ѧ�ڵڶ������������ۣ���ѧ���� ���ͣ���ѡ��
��ͼ�У�PΪ�����ɻ����Ļ������ر�K��V��A����aL��V��B����0��8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��ֱ�������A��B�и�����1molN2��3molH2��������ӦN2��g����3H2��g��  2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��
2NH3��g������H����92��4kJ/mol���ﵽƽ���V��B����0��6aL��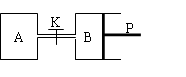
��B��N2��ת������ ��A��N2��ת���� ������ڡ��������ڡ���С�ڡ���B��N2��ת���ʡ������� ��
�ƴ�K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ ����˵�����ɣ�
��A��Ӧ�ﵽƽ��ʱ���ų�akJ������������A�г���2molNH3������ͬ�����´ﵽƽ��ʱ��������ΪbkJ����a��b�Ĺ�ϵ�� ��
��B�з�Ӧ�ﵽƽ���ֻ�ı�����������c��NH3�����ı���� ������ţ���
A�������¶� B������������1molAr��g��
C���������г���2molNH3 D��ѡ��Ч�����õĴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com