
��15�֣�������ң�G�����ڱ��������ҩ����ʹЧ�����ڲ���ҡ���ͼ�ǰ�����ҵ�һ���ϳ�·�ߡ�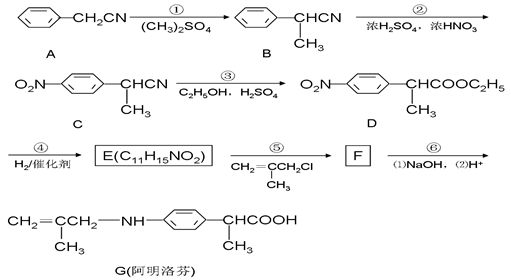
��1��E�Ľṹ��ʽΪ ���ڵķ�Ӧ���ͣ� ��
��2��D�к��������ŵ�����Ϊ �� ��
��3����Ӧ�ۿ��Կ�����������Ӧ���ܷ�Ӧ����һ�����������CN������ȫˮ�ⷴӦ�����Ȼ�����COOH������д���ڶ�����Ӧ�Ļ�ѧ����ʽ�� ��
��4������д�����ַ�������������E��ͬ���칹��Ľṹ��ʽ �� ��
������FeCl3��Һ������ɫ��Ӧ ���ܷ���ˮ�ⷴӦ�������ܷ���������Ӧ
�۱����ϵ�ȡ����ֻ��2�֣��ұ����ϵĺ˴Ź����������������շ�
��5�������йذ�����ң�G����˵����ȷ���ǣ� ��
| A������ʽΪC13H17NO2 | B�����ڰ����� |
| C���������ᷴӦ������ | D���ܷ���ȡ�����Ӿۡ���������ԭ��Ӧ |
��1��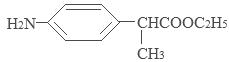 ��ȡ����Ӧ����2��������������
��ȡ����Ӧ����2��������������
��3�� ��4��
��4��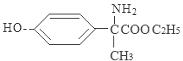 ��
��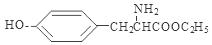 ��
��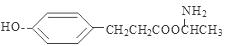 ;
; 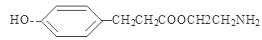 ;
; 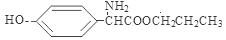 ;
;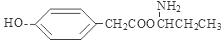 ;
;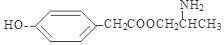 ;
;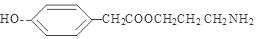 �ȡ�
�ȡ�
��5��A��C��D��
���������������1������D��E�ķ���ʽ��֪D������ԭ��Ӧ����E��D�����е���������ԭΪ������ E�Ľṹ��ʽΪ ������B��C�ķ��ӽṹ���Կ���B����ȡ����Ӧ��Ҳ��������Ӧ������C�����Ԣڵķ�Ӧ������ȡ����Ӧ����2����D�ķ��ӽṹ�еĺ��������ŵ�����Ϊ��������������3����Ӧ�ۿ��Կ�����������Ӧ���ܷ�Ӧ����һ�����������CN������ȫˮ�ⷴӦ�����Ȼ�����COOH�����ڶ�����ˮ��������Ȼ����Ҵ�����������Ӧ�����������÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
������B��C�ķ��ӽṹ���Կ���B����ȡ����Ӧ��Ҳ��������Ӧ������C�����Ԣڵķ�Ӧ������ȡ����Ӧ����2����D�ķ��ӽṹ�еĺ��������ŵ�����Ϊ��������������3����Ӧ�ۿ��Կ�����������Ӧ���ܷ�Ӧ����һ�����������CN������ȫˮ�ⷴӦ�����Ȼ�����COOH�����ڶ�����ˮ��������Ȼ����Ҵ�����������Ӧ�����������÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ����4��E�ṹ��ʽ��
����4��E�ṹ��ʽ�� ������ͬ���칹������FeCl3��Һ������ɫ��Ӧ���з��ǻ������ܷ���ˮ�ⷴӦ�����������ļ��������ܷ���������Ӧ˵�������Ǽ����γɵ������۱����ϵ�ȡ����ֻ��2�֣��ұ����ϵĺ˴Ź����������������շ壬����ǻ��������ڱ�������Ե�λ�ã�����������E��ͬ���칹��Ľṹ��ʽ�����е����ֿ�����
������ͬ���칹������FeCl3��Һ������ɫ��Ӧ���з��ǻ������ܷ���ˮ�ⷴӦ�����������ļ��������ܷ���������Ӧ˵�������Ǽ����γɵ������۱����ϵ�ȡ����ֻ��2�֣��ұ����ϵĺ˴Ź����������������շ壬����ǻ��������ڱ�������Ե�λ�ã�����������E��ͬ���칹��Ľṹ��ʽ�����е����ֿ����� ��
�� ��
��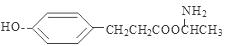 ;
; 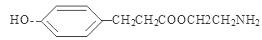 ;
;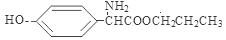 ;
;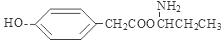 ;
;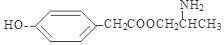 ;
;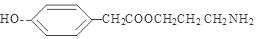 �ȡ���5��A���ɰ�����ң�G���Ľṹ��ʽ��֪�������ʽ��C13H17NO2 ����ȷ��B�����ڸ÷��Ӳ����а�����ֻ�����Ȼ������Բ����ڰ����ᣬ���� C���������ǰ�����Nԭ������һ�Թ¶Ե��ӣ������������ᷴӦ�����Σ���ȷ�� D�����������ʵķ����к����Ȼ��������ܷ���ȡ����Ӧ������̼̼˫�������Կ��Է����Ӿ۷�Ӧ��Ҳ�ܱ�������������ȼ�շ�Ӧ��Ҳ���������������ӳɷ�Ӧ���������ļӳɷ�Ӧ���Ƿ����˻�ԭ��Ӧ�����ѡ����A��C��D��
�ȡ���5��A���ɰ�����ң�G���Ľṹ��ʽ��֪�������ʽ��C13H17NO2 ����ȷ��B�����ڸ÷��Ӳ����а�����ֻ�����Ȼ������Բ����ڰ����ᣬ���� C���������ǰ�����Nԭ������һ�Թ¶Ե��ӣ������������ᷴӦ�����Σ���ȷ�� D�����������ʵķ����к����Ȼ��������ܷ���ȡ����Ӧ������̼̼˫�������Կ��Է����Ӿ۷�Ӧ��Ҳ�ܱ�������������ȼ�շ�Ӧ��Ҳ���������������ӳɷ�Ӧ���������ļӳɷ�Ӧ���Ƿ����˻�ԭ��Ӧ�����ѡ����A��C��D��
���㣺�����л���Ľṹ�����ʡ��ת������ѧ����ʽ��ͬ���칹�����д��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪���ԣ�H2SO4 >  >H2CO3>
>H2CO3>  > HCO3�����ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ���
> HCO3�����ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ��� ת��Ϊ
ת��Ϊ ����ѷ�����( )
����ѷ�����( )
| A����ϡH2SO4���Ⱥ���������NaOH��Һ |
| B����ϡH2SO4���Ⱥ���������Na2CO3��Һ |
| C����������NaOH��Һ���Ⱥ���ͨ������CO2 |
| D����������NaOH��Һ���Ⱥ��ټ�������H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��X�����Ǻ���һ������ʯ�ͻ���ˮƽ�ߵ͵ı�־��A��E��ΪX��ͬϵ�
��֪����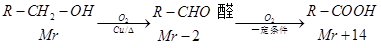
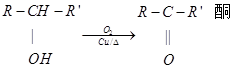
 (ͪ��R��R������������ͪ�����ٱ�����Ϊ���ᣬ����������R��R��������������ԭ��)
(ͪ��R��R������������ͪ�����ٱ�����Ϊ���ᣬ����������R��R��������������ԭ��)
�ڵͼ���ͨ������ˮ����ζ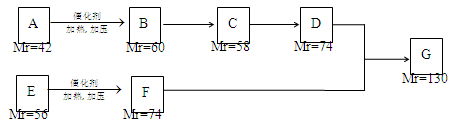
����ͼ����EΪֱ���ṹ��Fֻ�ܱ�����Ϊͪ����
��1��X�����ŽṹʽΪ �� A��B��Ӧ���� ��
��2��E���ܵĽṹ��ʽΪ ��B��G�й��������Ʒֱ��� ��
��3��A���γɵĸ߷��ӻ������dz���ʳƷ��װ����ԭ���ϣ�д���˸߾���Ľṹ��ʽ ��
��4��д��D��F��Ӧ����G�Ļ�ѧ����ʽ ��
��5����������������G��ͬ���칹�干�� �֣�������G����(�پ���ˮ����ζ������������ˮ��������Է�������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣��������� (idoxifene)������������������֢�����ĺϳ�·����ͼ��
��1��������A�ķ���ʽ�� ��1molA������� H2������Ӧ
��2����Ӧ���ͣ�B��C �� D��E
��3��D�к����������У� ��д���ƣ���
��4��������E�ܷ����ķ�Ӧ������ ��������ţ�
| A���ӳɷ�Ӧ | B��������Ӧ | C��ˮ�ⷴӦ | D���Ӿ۷�Ӧ |
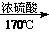 CH2=CH2
CH2=CH2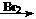 CH2Br-CH2Br
CH2Br-CH2Br�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��)A��G�����л�����ǵ�ת����ϵ���£�
��ش��������⣺
��1����֪��6��0g������E��ȫȼ������8.8gCO2��3.6gH2O��E������������������ܶ�Ϊ30����E�ķ���ʽΪ ��
��2��AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ
��3��д������ת���Ļ�ѧ����ʽ��
B��D
E+C��F
��4������������ת���õ���F�ж���ͬ���칹�壬ͬʱ��������������F��ͬ���칹���� �֡�
�ٱ�����ֻ��������λȡ����������һ��Ϊ�� ����F������ͬ�Ĺ����š�
����1molij���������������л��������������Һ��Ӧʱ����2mol�������ƣ��䷴Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A��B��C��D��E��F��G��H�ת����ϵ����ͼ��ʾ��5.2 g F����100 mL 1 mol/L NaOH��Һǡ����ȫ�кͣ�0.1 mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48 L CO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���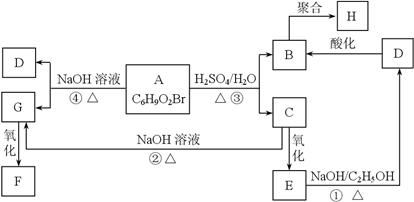
��1��д������C�еĹ����ŵ����ƣ� ��
��2��д������F��H�Ľṹ��ʽ��
F ��H ��
��3��д����Ӧ�١��ܵĻ�ѧ��Ӧ���ͣ��� ���� ��
��4��д���仯�١��۵Ļ�ѧ����ʽ��
��
��
��5��д����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ�������������칹����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣�����ʯ�͵��л�����ԭ��A�����������Ϊ����һ������ʯ�ͻ�����չˮƽ�ı�־��A���Է�������ת����
��֪��E�Ǿ��й���ζ���л�������ʽΪC4H8O2��F��һ�ָ߷��ӻ����
��1��A�ķ���ʽ��������������������C������������������������
��2��D�����еĹ���������������������֤���ù����ž������Եķ����� ������������
��3����Ӧ�۵Ļ�ѧ����ʽ�� ����Ӧ�ܵ������� ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϩ��ʯ�ͻ�������Ҫ�Ļ���ԭ�ϣ���������¿�ͼ�ش�
41.��ʯ��Ϊԭ�ϵ�ϵ�л������������У��õ�������ϩ����Ҫ������_______��ѡ����ţ���
a. ˮ�� b. ���� c. �ѽ� d. �ѻ�
42.�л���A�׳�______________�����еĹ�����������_________________.
43.B�ķ���ʽΪC2H4O2���봿�Ӧ�����ɶ�����̼���壬д����ӦA��B��C�Ļ�ѧ
����ʽ___________________________________________________________ ���л����ýṹ��ʽ��ʾ�����÷�Ӧ����Ϊ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�߷�����άE�㷺����ͨѶ����������������÷�����AΪԭ�����ϳɣ��ϳ�·�����£�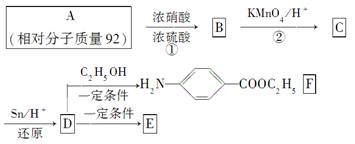
��ش�
(1)д���ṹ��ʽ��A__________��B____________��
C____________��
(2)д����Ӧ���ͣ���__________����_______________________________��
(3)д��D��E�ۺϵĻ�ѧ����ʽ___________________��
(4)���л�����������F������ѧ��Ӧ����________��
a��HCl b��NaCl c��Na2CO3 d��NaOH
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com