
��13�֣�
��1��ij��ѧʵ��С����г�������һƿʳ�ð״ף���CH3COOH������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����Ũ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9 ���±���4�ֳ���ָʾ�� �ı�ɫ��Χ��
�ı�ɫ��Χ��
| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
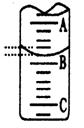
A��ʵ�� ����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ����� ����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ����� |
| B���ζ�ǰ�ζ��ܼ��������ݣ��ζ��������첿�ֳ�����Һ |
| C��ʢװ�״���Һ�ĵζ���������ˮϴ����δ�ð״���Һ��ϴ |
| D���μ�NaOH��Һʱ��δ������տ�����Һ��ɫ������ֹͣ�ζ� |
 �����ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ���
�����ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ��� �ζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\
�ζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\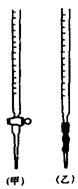

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011�긣����Ϫһ�С��ݰ�һ�С�������ѧ�߶���ѧ����ĩ������ѧ�� ���ͣ�ʵ����
��13�֣�
��1��ij��ѧʵ��С����г�������һƿʳ�ð״ף���CH3COOH������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����Ũ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9 ���±���4�ֳ���ָʾ���ı�ɫ��Χ��
|
ָʾ�� |
ʯ�� |
���� |
���� |
��̪ |
|
��ɫ��Χ��pH�� |
5.0��8.0 |
3.1��4.4 |
4.4��6.2 |
8.2��10.0 |
�ٸ�ʵ��Ӧѡ�� ��ָʾ����
����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ mL��

��Ϊ��Сʵ������ͬѧһ������������ʵ�飬����ÿ
����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ��
�����¼���£�

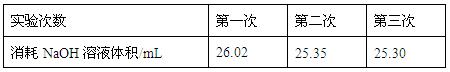
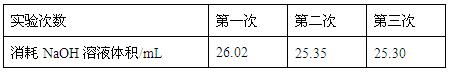
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A��ʵ�����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ��������첿�ֳ�����Һ
C��ʢװ�״���Һ�ĵζ���������ˮϴ����δ�ð״���Һ��ϴ
D���μ�NaOH��Һʱ��δ������տ�����Һ��ɫ������ֹͣ�ζ�
��2���Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ���
2MnO4����5H2C2O4��6H�� 2Mn2����10CO2����8H2O
����ͬѧ����˵ζ��ķ����ⶨxֵ��
��ȡ1.260 g�����ᾧ�壬���Ƴ�100.00 mLˮ��ҺΪ����Һ��
ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
��Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�ٵζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\

�� ��ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��1��ij��ѧʵ��С����г�������һƿʳ�ð״ף���CH3COOH������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����Ũ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9 ���±���4�ֳ���ָʾ���ı�ɫ��Χ��
| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
�ٸ�ʵ��Ӧѡ�� ��ָʾ����
����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ mL��
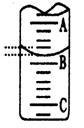
��Ϊ��Сʵ������ͬѧһ������������ʵ�飬����ÿ
����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ��
�����¼���£�
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A��ʵ�����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ��������첿�ֳ�����Һ
C��ʢװ�״���Һ�ĵζ���������ˮϴ����δ�ð״���Һ��ϴ
D���μ�NaOH��Һʱ��δ������տ�����Һ��ɫ������ֹͣ�ζ�
��2���Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ���
2MnO4����5H2C2O4��6H�� 2Mn2����10CO2����8H2O
����ͬѧ����˵ζ��ķ����ⶨxֵ��
��ȡ1.260 g�����ᾧ�壬���Ƴ�100.00 mLˮ��ҺΪ����Һ��
ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
��Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�� �ζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\
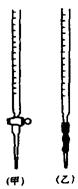
�� ��ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��13�֣�
��1��ij��ѧʵ��С����г�������һƿʳ�ð״ף���CH3COOH������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����Ũ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9 ���±���4�ֳ���ָʾ���ı�ɫ��Χ��
| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
�ٸ�ʵ��Ӧѡ�� ��ָʾ����
����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ mL��
��Ϊ��Сʵ������ͬѧһ������������ʵ�飬����ÿ
����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ��
�����¼���£�
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A��ʵ�����ʱ�����ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ��������첿�ֳ�����Һ
C��ʢװ�״���Һ�ĵζ���������ˮϴ����δ�ð״���Һ��ϴ
D���μ�NaOH��Һʱ��δ������տ�����Һ��ɫ������ֹͣ�ζ�
��2���Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ���������ϵ�֪������������ˮ���л�ԭ�ԣ�����������KMnO4��Һ���еζ���
2MnO4����5H2C2O4��6H�� 2Mn2����10CO2����8H2O
����ͬѧ����˵ζ��ķ����ⶨxֵ��
��ȡ1.260 g�����ᾧ�壬���Ƴ�100.00 mLˮ��ҺΪ����Һ��
ȡ25.00mL����Һ������ƿ�У��ټ���������ϡH2SO4��
��Ũ��Ϊ0.1000mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�ٵζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�\
�� ��ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x=
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com