
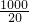 =1.0675L��
=1.0675L��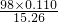 ��100%��70.6%��
��100%��70.6%��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ����һ�и�����һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
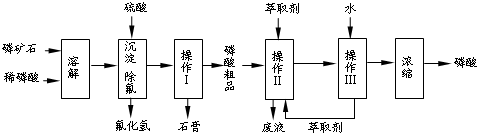
�����16�֣���ʯ����Ҫ�ɷ���Ca5F(PO4)3��������MgO��Fe2O3�����ʡ���ҵ������ʯΪԭ���Ʊ�H3PO4�ij����������£�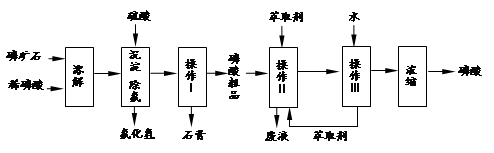
��֪��Ca5F(PO4)3+ 7H3PO4��5Ca (H2PO4)2+HF
��1���������ַ�����ʵ�����ܽ���ʯ________����ܡ����ܡ����ò���������ԭ����_____________________________________��
��2���������������___________
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ���������_______________________���Ʋ����ȡ��һ�����е�������_________��
a. ����ȡ����ˮ�������� b. ��ͬ�����£�����ȡ�����ܶȱ�ˮС
c. �����ڸ���ȡ���е��ܽ�Ⱥ�С d. ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬����__________________�ȸ���Ʒ��������˵������һ�ָ���Ʒ����;____________________________________��
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ���____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 5.1 ��������Եĸ�����ϰ���������棩 ���ͣ�ѡ����
������������ȷ����(˫ѡ)(����)
A���ؽ������л������������Ⱦ����ʹ�����ữ
B�����������Է��ϣ�����ʹ�ò�����������������Ӱ��
C���̬���ʲ������ľ�һ��ʹ���������ˮ���й�
D������ʯ(��Ҫ�ɷ��������)�ӹ��ɹ��������Ϊ�������Ч�ɷֵ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
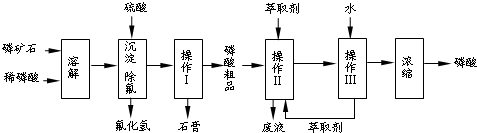
�����16�֣���ʯ����Ҫ�ɷ���Ca5F(PO4)3��������MgO��Fe2O3�����ʡ���ҵ������ʯΪԭ���Ʊ�H3PO4�ij����������£�
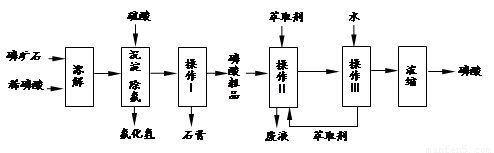
��֪��Ca5F(PO4)3+ 7H3PO4��5Ca (H2PO4)2+HF
��1���������ַ�����ʵ�����ܽ���ʯ________����ܡ����ܡ����ò���������ԭ����_____________________________________��
��2���������������___________
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ���������_______________________���Ʋ����ȡ��һ�����е�������_________��
a. ����ȡ����ˮ�������� b. ��ͬ�����£�����ȡ�����ܶȱ�ˮС
c. �����ڸ���ȡ���е��ܽ�Ⱥ�С d. ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬����__________________�ȸ���Ʒ��������˵������һ�ָ���Ʒ����;____________________________________��
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ���____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com