
��7�֣��йش����Ĵ������������ǿ��Դӡ��Ҵ���ʵ�顱�еõ�һЩ��ʶ��ij��ʦ�������ͼ��ʾװ�ã��г�װ����ʡ�ԣ����Իش��������⣺
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ��___________________________ ��
��2����Ӧһ��ʱ�������ȥ�ƾ��Ʒ�Ӧ���ܼ������У���˵�����Ҵ���������Ӧ��_________________(����ȡ������ȡ�)��Ӧ��
��3��ʵ�������D��ͭ˿���к�ɫ�ͺ�ɫ������ֵ��������û�ѧ����ʽ����ԭ������٣���ɫ��� ɫ��___________________________________________��
ɫ��___________________________________________��
����ڣ���ɫ���ɫ��____________________________________________
����Щʵ�������п�����ʶ��ʵ������д���__________����μӡ����μӡ�����ѧ��Ӧ��
��4��װ��B��F�����÷ֱ���
B:______________________________________ ,
F:_________________________________ ��
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| Cu |
| �� |
| Cu |
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| Cu |
| �� |
| Cu |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ�ڵ�һ�μ�⻯ѧ�Ծ��������棩 ���ͣ������
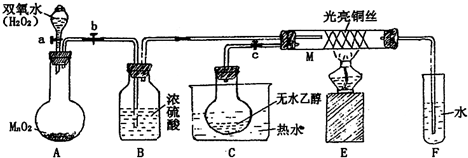
�йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ����ͼ��װ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ������

��1��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________��B�����ã�____________�� C�����ã�____________��
��2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ��______________________________________
��3����M���пɹ۲쵽������Ϊ_______________�����п���ʶ����ʵ������д���___ __ ____����μӡ����μӡ����˻�ѧ��Ӧ
��4����֤�Ҵ�����������Լ��� ����д����Ӧ�Ļ�ѧ����ʽ ��
��5�����Թ�F���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� ����д��ĸ����
A���Ȼ�����Һ B���� C��̼��������Һ D�����Ȼ�̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��7�֣��йش����Ĵ������������ǿ��Դӡ��Ҵ���ʵ�顱�еõ�һЩ��ʶ��ij��ʦ�������ͼ��ʾװ�ã��г�װ����ʡ�ԣ����Իش��������⣺

��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ��___________________________ ��
��2����Ӧһ��ʱ�������ȥ�ƾ��Ʒ�Ӧ���ܼ������У���˵�����Ҵ���������Ӧ��_________________(����ȡ������ȡ�)��Ӧ��
��3��ʵ�������D��ͭ˿���к�ɫ�ͺ�ɫ������ֵ��������û�ѧ����ʽ����ԭ������٣���ɫ���ɫ��___________________________________________��
����ڣ���ɫ���ɫ��____________________________________________
����Щʵ�������п�����ʶ��ʵ������д���__________����μӡ����μӡ�����ѧ��Ӧ��
��4��װ��B��F�����÷ֱ���
B:______________________________________ ,
F:_________________________________ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com