
Ρ≥÷÷¥÷―Έ÷–Κ§”–Ρύ…≥ΓΔCa2ΘΪΓΔMg2ΘΪΓΔFe3ΘΪΓΔSOΒ»‘”÷ ΓΘΡ≥Ά§―ß‘Ύ Β―ι “÷–…ηΦΤΝΥ”Ο’β÷÷¥÷―Έ÷Τ±ΗΨΪ―ΈΒΡΖΫΑΗ»γœ¬(”Ο”Ύ≥ΝΒμΒΡ ‘ΦΝ…‘ΙΐΝΩ)ΘΚ

«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
(1)ΈΣ≤ΌΉςΔτ―Γ‘ώΥυ–η“«Τς(”Ο±ξΚ≈Ή÷ΡΗΧν–¥)ΘΚ________ΓΘ
AΘ°…’±≠ΓΓBΘ° ‘ΙήΓΓCΘ°≤ΘΝßΑτΓΓDΘ°Ζ÷“Κ¬©ΕΖΓΓEΘ°¬©ΕΖΓΓFΘ°ΨΤΨΪΒΤΓΓ
GΘ°’τΖΔΟσ
(2)≤ΌΉςΔσ÷–≥Θ”ΟNa2CO3»ή“ΚΓΔNaOH»ή“ΚΓΔBaCl2»ή“ΚΉςΈΣ≥ΐ‘” ‘ΦΝΘ§‘ρΦ”»κ≥ΐ‘” ‘ΦΝΒΡΥ≥–ρΈΣΘΚNaOH»ή“ΚΓζ________Γζ________ΓΘ
(3)≤ΌΉςΔσ÷–Θ§≈–ΕœΦ”»κBaCl2“―ΙΐΝΩΒΡΖΫΖ® «_________________________ΓΘ
(4)≤ΌΉςΔθ”Π―Γ‘ώΒΡΥα «________Θ§»τΫΪ≤ΌΉςΔθ”κ≤ΌΉςΔτΒΡœ»ΚσΥ≥–ρΕ‘ΒςΘ§ΫΪΜαΕ‘ Β―ιΫαΙϊ≤ζ…ζΒΡ”Αœλ «__________________________________________ΓΘ
(5)≤ΌΉςΔω «________(―Γ‘ώΚœάμ≤ΌΉςΒΡΟϊ≥ΤΘ§”Ο±ξΚ≈Ή÷ΡΗΑ¥≤ΌΉςœ»ΚσΥ≥–ρΧν–¥)ΓΘ
aΘ°Ιΐ¬ΥΓΔœ¥Β” bΘ°’τΖΔΓΔ≈®Υθ cΘ°ίΆ»ΓΓΔΖ÷“Κ dΘ°ά以ΓΔΫαΨß
ΓΓ(1)ACE (2)BaCl2»ή“ΚΓΓNa2CO3»ή“Κ
(3)»ΓΥυΒΟ»ή“ΚΒΡ…œ≤ψ«ε“Κ”Ύ ‘Ιή÷–Θ§‘ΌΒΈ»κ…ΌΝΩBaCl2»ή“ΚΘ§»τ»ή“ΚΈ¥±δΜκΉ«Θ§‘ρ±μΟςBaCl2“―ΙΐΝΩ
(4)―ΈΥαΓΓ‘ΎΥα–‘ΧθΦΰœ¬Θ§Μα”–≤ΩΖ÷≥ΝΒμ»ήΫβΘ§¥”Εχ”ΑœλΨΪ―ΈΒΡ¥ΩΕ»
(5)bda
ΓΨΫβΈωΓΩΓΓ(1)≤ΌΉςΔτ «Ιΐ¬ΥΓΘ(2)≥ΐ‘” ‘ΦΝ «ΙΐΝΩΒΡΘ§ΈΣΝΥ≥ΐ»ΞΙΐΝΩΒΡBa2ΘΪΘ§–η“Σ‘ΎΦ”»κBaCl2»ή“Κ÷°ΚσΦ”»κNa2CO3»ή“ΚΓΘ(3)BaCl2ΙΐΝΩΚσΘ§»ή“Κ÷–≤Μ¥φ‘ΎSO42ΓΣΘ§»Γ…œ≤ψ«ε“ΚΦ”»κBaCl2»ή“ΚΚσ≤ΜΜα≤ζ…ζ≥ΝΒμΓΘ(4)Φ”»κΒΡNa2CO3»ή“Κ «ΙΐΝΩΒΡΘ§Φ”»κ―ΈΥαΒςΫΎ»ή“ΚΒΡpHΦ¥Ω…≥ΐ»ΞCO32ΓΣΘ§ΒΟΒΫ¥ΩΨΜΒΡ ≥―ΈΥ°ΓΘ»τ‘ΎΙΐ¬Υ«ΑΥαΜ·Θ§‘ρ‘ΎΥα–‘ΧθΦΰœ¬Θ§Μα”–≤ΩΖ÷≥ΝΒμ»ήΫβΘ§¥”Εχ”ΑœλΨΪ―ΈΒΡ¥ΩΕ»ΓΘ(5)¥” ≥―ΈΥ°ΒΟΒΫ ≥―ΈΨßΧε–η“ΣΨ≠Ιΐ’τΖΔΓΔ≈®ΥθΓΔά以ΓΔΫαΨßΓΔΙΐ¬ΥΓΔœ¥Β”Β»Ιΐ≥ΧΓΘ


 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ5Ϋ≤Μ·―ßΖ¥”Π”κΡήΝΩΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“―÷Σ““»≤”κ±Ϋ’τΤχΆξ»Ϊ»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ»γœ¬ΘΚ
ΔΌC2H2(g)ΘΪ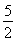 O2(g)®DΓζ2CO2(g)ΘΪH2O(l) ΠΛHΘΫΘ≠1 300 kJΓΛmolΘ≠1
O2(g)®DΓζ2CO2(g)ΘΪH2O(l) ΠΛHΘΫΘ≠1 300 kJΓΛmolΘ≠1
ΔΎC6H6(g)ΘΪ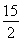 O2(g)®DΓζ6CO2(g)ΘΪ3H2O(l) ΠΛHΘΫΘ≠3 295 kJΓΛmolΘ≠1
O2(g)®DΓζ6CO2(g)ΘΪ3H2O(l) ΠΛHΘΫΘ≠3 295 kJΓΛmolΘ≠1
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)ΓΘ
AΘ°1 mol C2H2(g)Άξ»Ϊ»Φ…’…ζ≥…ΤχΧ§Υ° ±Ζ≈»»¥σ”Ύ1 300 kJ
BΘ°1 mol C6H6(l)Άξ»Ϊ»Φ…’…ζ≥…“ΚΧ§Υ° ±Ζ≈»»¥σ”Ύ3 295 kJ
CΘ°œύΆ§ΧθΦΰœ¬Θ§Β»÷ ΝΩΒΡC2H2(g)”κC6H6(g)Άξ»Ϊ»Φ…’Θ§C6H6(g)Ζ≈»»ΗϋΕύ
DΘ°C2H2(g)»ΐΨέ…ζ≥…C6H6(g)ΒΡΙΐ≥Χ τ”ΎΖ≈»»Ζ¥”Π
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ1Ϋ≤Έο÷ ΒΡΉι≥…–‘÷ ΚΆΖ÷άύΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
ΙηΦΑΤδΜ·ΚœΈοΕ‘»Υάύœ÷¥ζΈΡΟςΒΡΖΔ’ΙΨΏ”–ΧΊ βΙ±œΉΓΘ«κΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
(1)Ιη‘≠Ή”ΒΡΫαΙΙ Ψ“βΆΦΘΚ________ΓΘ
(2)œ¬Ν–ΈοΤΖΜρ…η±ΗΥυ”ΟΒΡ≤ΡΝœ τ”ΎΙηΥα―ΈΒΡ «________ΓΘ
ΔΌ≥ΛΫ≠»ΐœΩΥ°Ρύ¥σΑ”ΓΓΔΎ ·”ΔΙβΒΦœΥΈ§ΓΓΔέΧ’¥…έαέω
ΔήΤ’Ά®≤ΘΝßΓΓΔίΙηΧΪ―τΡήΒγ≥Ί
AΘ°ΔΌΔΎΔί BΘ°ΔέΔήΔί CΘ°ΔΎΔέΔή DΘ°ΔΌΔέΔή
(3)≥ΘΈ¬œ¬Θ§SiCl4ΈΣ“ΚΧ§Θ§Ζ–ΒψΈΣ57.6 ΓφΘ§‘ΎΩ’Τχ÷–ΟΑΑΉΈμΓΘ÷Τ±ΗΗΏ¥ΩΕ»ΙηΒΡ÷–Φδ≤ζΈοSiCl4÷–»ή”–“ΚΧ§‘”÷ Θ§»τ“ΣΒΟΒΫΗΏ¥ΩΕ»SiCl4Θ§”Π≤…”ΟΒΡΖΫΖ® «________ΘΜ”ΟΜ·―ßΖΫ≥Χ ΫΦΑ±Ί“ΣΈΡΉ÷Ϋβ ΆSiCl4‘ΎΩ’Τχ÷–ΟΑΑΉΈμΒΡ‘≠“ρΘΚ
___________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ14Ϋ≤Έο÷ ΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Έ“Ιζ≤ΩΖ÷≥« –Μ“ω≤Χλ’Φ»ΪΡξ“ΜΑκΘ§“ΐΤπΜ“ω≤ΒΡPM2.5ΈΔœΗΝΘΉ”ΑϋΚ§(NH4)2SO4ΓΔNH4NO3ΓΔ”–ΜζΩ≈ΝΘΈοΦΑ―ο≥ΨΒ»ΓΘΆ®Ιΐ≤βΕ®Μ“ω≤÷––ΩΒ»÷ΊΫπ τΒΡΚ§ΝΩΘ§Ω…÷ΣΡΩ«Α‘λ≥…Έ“ΙζΜ“ω≤ΧλΤχΒΡ‘≠“ρ÷ς“Σ «ΫΜΆ®Έέ»ΨΓΘ
(1)Zn2ΘΪ‘ΎΜυΧ§ ±ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ_______________________________ΓΘ
(2)SO42ΓΣΒΡΩ’ΦδΙΙ–Ά «________(”ΟΈΡΉ÷Οη ω)ΓΘ
(3)PM2.5ΗΜΚ§¥σΝΩΒΡ”–ΕΨΓΔ”–ΚΠΈο÷ Θ§“Ή“ΐΖΔΕΰ¥ΈΙβΜ·―ß―ΧΈμΈέ»ΨΘ§ΙβΜ·―ß―ΧΈμ÷–Κ§”–NOxΓΔO3ΓΔCH2===CHΓΣCHOΓΔHCOOHΓΔ (PAN)Β»Εΰ¥ΈΈέ»ΨΈοΓΘ
(PAN)Β»Εΰ¥ΈΈέ»ΨΈοΓΘ
ΔΌœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________ΘΜ
aΘ°N2OΫαΙΙ ΫΩ…±μ ΨΈΣN===N===O
bΘ°O3Ζ÷Ή”≥ ÷±œΏ–Έ
cΘ°CH2===CHΓΣCHOΖ÷Ή”÷–ΧΦ‘≠Ή”Ψυ≤…”Οsp2‘”Μ·
dΘ°œύΆ§―Ι«Ωœ¬Θ§HCOOHΖ–Βψ±»CH3OCH3ΗΏΘ§ΥΒΟς«Α’Ώ «ΦΪ–‘Ζ÷Ή”Θ§Κσ’Ώ «Ζ«ΦΪ–‘Ζ÷Ή”
ΔΎ1 mol PAN÷–Κ§Π“Φϋ ΐΡΩΈΣ________ΘΜ

ΔέNOΡή±ΜFeSO4»ή“ΚΈϋ ’…ζ≥…≈δΚœΈο[Fe(NO)(H2O)5]SO4Θ§ΗΟ≈δΚœΈο÷––ΡάκΉ”ΒΡ≈δΈΜ ΐΈΣ________(Χν ΐΉ÷)ΓΘ
(4)≤βΕ®¥σΤχ÷–PM2.5ΒΡ≈®Ε»ΖΫΖ®÷°“Μ «Π¬?…δœΏΈϋ ’Ζ®Θ§Π¬?…δœΏΖ≈…δ‘¥Ω…”Ο85KrΓΘ“―÷ΣKrΨßΧεΒΡΨßΑϊΫαΙΙ»γΆΦΥυ ΨΘ§…ηΨßΧε÷–”κΟΩΗωKr‘≠Ή”œύΫτΝΎΒΡKr‘≠Ή””–mΗωΘ§ΨßΑϊ÷–Κ§Kr‘≠Ή”ΈΣnΗωΘ§‘ρm/nΘΫ________(Χν ΐΉ÷)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ13Ϋ≤ Β―ιΖΫΑΗ…ηΦΤ”κΤάΦέΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
ΧΦΥαΡΤΓΣΙΐ―θΜ·«βΦ”ΚœΈο(aNa2CO3ΓΛbH2O2)ΨΏ”–Τ·ΑΉΓΔ…±ΨζΉς”ΟΓΘ Β―ι “”ΟΓΑ¥ΦΈωΖ®Γ±÷Τ±ΗΗΟΈο÷ ΒΡ Β―ι≤Ϋ÷η»γœ¬ΘΚ
ΒΎ1≤ΫΘΚ»Γ ΝΩΧΦΥαΡΤ»ήΫβ”Ύ“ΜΕ®ΝΩΥ°άοΘ§ΒΙ»κ…’ΤΩ÷–ΘΜ‘ΌΦ”»κ…ΌΝΩΈ»Ε®ΦΝ(MgCl2ΚΆNa2SiO3)Θ§ΫΝΑηΨυ‘»ΓΘ
ΒΎ2≤ΫΘΚΫΪ ΝΩ30%ΒΡH2O2»ή“Κ‘ΎΫΝΑηΉ¥Χ§œ¬ΒΈ»κ…’ΤΩ÷–Θ§”Ύ15 ΓφΉσ”“Ζ¥”Π1 hΓΘ
ΒΎ3≤ΫΘΚΖ¥”ΠΆξ±œΚσ‘ΌΦ”»κ ΝΩΈόΥ°““¥ΦΘ§Ψ≤÷ΟΓΔΫαΨßΘ§Ιΐ¬ΥΓΔΗ…‘οΒΟ≤ζΤΖΓΘ
(1)ΒΎ1≤Ϋ÷–Θ§Έ»Ε®ΦΝ”κΥ°Ζ¥”Π…ζ≥…ΝΫ÷÷≥ΘΦϊΒΡΡ―»ήΈοΘ§ΤδΜ·―ßΖΫ≥Χ ΫΈΣ___________________________________________________________ΓΘ
(2)ΒΎ2≤Ϋ÷–Θ§Ζ¥”Π±Θ≥÷ΈΣ15 ΓφΉσ”“Ω…≤…»ΓΒΡ¥κ © «_____________________
___________________________________________________ΓΘ
(3)ΒΎ3≤Ϋ÷–Θ§ΈόΥ°““¥ΦΒΡΉς”Ο «____________________________________ΓΘ
(4)H2O2ΒΡΚ§ΝΩΩ…ΚβΝΩ≤ζΤΖΒΡ”≈Ν”ΓΘœ÷≥Τ»Γm g(‘Φ0.5 g)―υΤΖΘ§”Ο–¬÷σΖ–ΙΐΒΡ’τΝσΥ°≈δ÷Τ≥…250 mL»ή“ΚΘ§»Γ25.0 mL”ΎΉΕ–ΈΤΩ÷–Θ§œ»”ΟœΓΝρΥαΥαΜ·Θ§‘Ό”Οc molΓΛLΘ≠1 KMnO4»ή“ΚΒΈΕ®÷Ν÷’ΒψΓΘ
ΔΌ≈δ÷Τ250 mL»ή“ΚΥυ–ηΒΡ≤ΘΝß“«Τς”–…’±≠ΓΔ≤ΘΝßΑτΓΔΝΩΆ≤________ΓΔ________ΘΜ
ΔΎΒΈΕ®÷’ΒψΙέ≤λΒΫΒΡœ÷œσ «______________________________________ΓΘ
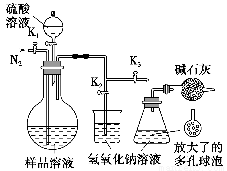
(5)Ω…ΡΘΡβ”Ο’τΝσΖ®≤βΕ®―υΤΖ÷–ΧΦΥαΡΤΒΡΚ§ΝΩΓΘΉΑ÷Ο»γ”“ΆΦΥυ Ψ(Φ”»»ΚΆΙΧΕ®ΉΑ÷Ο“―¬‘»Ξ)Θ§ Β―ι≤Ϋ÷η»γœ¬ΘΚ
≤Ϋ÷η1ΘΚΑ¥”“ΆΦΥυ ΨΉιΉΑ“«ΤςΘ§Φλ≤ιΉΑ÷ΟΤχΟή–‘ΓΘ
≤Ϋ÷η2ΘΚΉΦ»ΖΝΩ»Γ(4)÷–Υυ≈δ»ή“Κ50 mL”Ύ…’ΤΩ÷–ΓΘ
≤Ϋ÷η3ΘΚΉΦ»ΖΝΩ»Γ40.00 mL‘Φ0.2 molΓΛLΘ≠1 NaOH»ή“ΚΝΫΖίΘ§Ζ÷±πΉΔ»κ…’±≠ΚΆΉΕ–ΈΤΩ÷–ΓΘ
≤Ϋ÷η4ΘΚ¥ρΩΣΜν»ϊK1ΓΔK2Θ§ΙΊ±’Μν»ϊK3ΜΚΜΚΆ®»κΒΣΤχ“ΜΕΈ ±ΦδΚσΘ§ΙΊ±’K1ΓΔK2Θ§¥ρΩΣK3ΘΜΨ≠Ζ÷“Κ¬©ΕΖœρ…’ΤΩ÷–Φ”»κ10 mL 3 molΓΛLΘ≠1ΝρΥα»ή“ΚΓΘ
≤Ϋ÷η5ΘΚΦ”»»÷Ν…’ΤΩ÷–ΒΡ“ΚΧεΖ–ΧΎΘ§’τΝσΘ§≤Δ±Θ≥÷“ΜΕΈ ±ΦδΓΘ
≤Ϋ÷η6ΘΚΨ≠K1‘ΌΜΚΜΚΆ®»κΒΣΤχ“ΜΕΈ ±ΦδΓΘ
≤Ϋ÷η7ΘΚœρΉΕ–ΈΤΩ÷–Φ”»κΥαΦν÷Η ΨΦΝΘ§”Οc1 molΓΛLΘ≠1 H2SO4±ξΉΦ»ή“ΚΒΈΕ®÷Ν÷’ΒψΘ§œϊΚΡH2SO4±ξΉΦ»ή“ΚV1 mLΓΘ
≤Ϋ÷η8ΘΚΫΪ Β―ι≤Ϋ÷η1ΓΪ7÷ΊΗ¥ΝΫ¥ΈΓΘ
ΔΌ≤Ϋ÷η3÷–Θ§ΉΦ»Ζ“Τ»Γ40.00 mL NaOH»ή“ΚΥυ–η“Σ Ι”ΟΒΡ“«Τς «________ΘΜ
ΔΎ≤Ϋ÷η1ΓΪ7÷–Θ§»Ζ±Θ…ζ≥…ΒΡΕΰ―θΜ·ΧΦ±Μ«β―θΜ·ΡΤ»ή“ΚΆξ»ΪΈϋ ’ΒΡ Β―ι≤Ϋ÷η «________(Χν–ρΚ≈)ΘΜ
ΔέΈΣΜώΒΟ―υΤΖ÷–ΧΦΥαΡΤΒΡΚ§ΝΩΘ§ΜΙ–η≤Ι≥δΒΡ Β―ι «______________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ12Ϋ≤Μ·―ß Β―ιΜυ¥ΓΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–”–ΙΊ Β―ιΉΑ÷ΟΒΡΥΒΖ®÷–’ΐ»ΖΒΡ «(ΓΓΓΓ)ΓΘ

AΘ°”ΟΆΦ1ΉΑ÷Ο÷Τ»ΓΗ…‘ο¥ΩΨΜΒΡNH3
BΘ°”ΟΆΦ2ΉΑ÷Ο÷Τ±ΗFe(OH)2≤ΔΡήΫœ≥Λ ±ΦδΙέ≤λΤδ―’…Ϊ
CΘ°”ΟΆΦ3ΉΑ÷ΟΩ…“‘Άξ≥…ΓΑ≈γ»ΣΓ± Β―ι
DΘ°”ΟΆΦ4ΉΑ÷Ο≤βΝΩCu”κœΓœθΥαΖ¥”Π≤ζ…ζΤχΧεΒΡΧεΜΐ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ11Ϋ≤”–ΜζΜ·―ßΜυ¥ΓΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
ύ≠ΝρΤΫΩ…”Ο”ΎΨΪ…ώΦ≤≤ΓΒΡ÷ΈΝΤΘ§ΥϋΒΡΚœ≥…¬ΖœΏ»γœ¬ΘΚ

(1)–¥≥ωC13H9NO4S÷–Υυ”–Κ§―θΙΌΡήΆ≈ΒΡΟϊ≥ΤΘΚ________ΓΘ
(2)A τ”ΎΧΰΘ§«“œύΕ‘Ζ÷Ή”÷ ΝΩ «54Θ§–¥≥ωAΒΡΫαΙΙΦρ ΫΘΚ____________ΓΘ
(3)Ζ¥”ΠΔΌΓΪΔί÷– τ”Ύ»Γ¥ζΖ¥”ΠΒΡ”–________(Χν–ρΚ≈)ΓΘ
–¥≥ωΖ¥”ΠΔΏΒΡΜ·―ßΖΫ≥Χ ΫΘΚ__________________________________________ΓΘ
(4)Νς≥Χ÷–…ηΦΤΖ¥”ΠΔίΚΆΔΏΒΡΡΩΒΡ «___________________________________ΓΘ
(5)Έο÷ CΒΡΆ§Ζ÷“λΙΙΧε”–Εύ÷÷Θ§Τδ÷–Φ»Κ§”–τ«ΜυΘ§”÷Κ§”–»©ΜυΒΡΆ§Ζ÷“λΙΙΧε”–________÷÷ΓΘ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ10Ϋ≤Ϋπ τ‘ΣΥΊΦΑΤδΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ’ΐ»ΖΒΡ «(ΓΓΓΓ)ΓΘ
AΘ°œΓΝρΥα”κΧζΖ¥”ΠΘΚ2FeΘΪ6HΘΪ=2Fe3ΘΪΘΪ3H2Γϋ
BΘ°2 molΓΛLΘ≠1ΒΡAlCl3»ή“ΚΚΆ7 molΓΛLΘ≠1ΒΡNaOH»ή“ΚΒ»ΧεΜΐΨυ‘»ΜλΚœΘΚ2Al3ΘΪΘΪ7OHΘ≠=Al(OH)3ΓΐΘΪAlO2ΓΣΘΪ2H2O
CΘ°Ba(OH)2»ή“Κ÷–Φ”»κ…ΌΝΩΒΡNaHSO4»ή“ΚΘΚBa2ΘΪΘΪ2OHΘ≠ΘΪ2HΘΪΘΪSO42ΓΣ=BaSO4ΓΐΘΪ2H2O
DΘ°NaHCO3ΒΡΥ°ΓΨΫβΈωΓΩ
HCO3ΓΣΘΪH2O CO32ΓΣΘΪH3OΘΪ
CO32ΓΣΘΪH3OΘΪ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΧαΖ÷―ΒΝΖ Ή®Χβ7Υ°»ή“Κ÷–ΒΡάκΉ”ΤΫΚβΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“Έ¬ ±Θ§œρ20 mL 0.1 molΓΛLΘ≠1¥ΉΥα»ή“Κ÷–≤ΜΕœΒΈ»κ0.1 molΓΛLΘ≠1 NaOH»ή“ΚΘ§»ή“ΚΒΡpH±δΜ·«ζœΏ»γœ¬ΆΦΥυ ΨΓΘ‘ΎΒΈΕ®Ιΐ≥Χ÷–Θ§œ¬Ν–ΙΊ”Ύ»ή“Κ÷–άκΉ”≈®Ε»¥σ–ΓΙΊœΒΒΡΟη ωΘ§≤Μ’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©ΓΘ

AΘ°aΒψ ±ΘΚcΘ®CH3COOHΘ©>cΘ®NaΘΪΘ©>cΘ®CH3COOΘ≠Θ©>cΘ®HΘΪΘ©>cΘ®OHΘ≠Θ©
BΘ°bΒψ ±ΘΚcΘ®NaΘΪΘ©ΘΫcΘ®CH3COOΘ≠Θ©>cΘ®HΘΪΘ©ΘΫcΘ®OHΘ≠Θ©
CΘ°cΒψ ±ΘΚcΘ®HΘΪΘ©ΘΫcΘ®CH3COOHΘ©ΘΪcΘ®OHΘ≠Θ©
DΘ°dΒψ ±ΘΚcΘ®NaΘΪΘ©>cΘ®CH3COOΘ≠Θ©>cΘ®OHΘ≠Θ©>cΘ®HΘΪΘ©
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com