
��֪����25ʱH2O![]() H++OH- KW=10-14
H++OH- KW=10-14
HAC![]() H++A
H++A![]() Ka=1.8��10-5
Ka=1.8��10-5
��1��������ˮ���ƽ�ⳣ��Kh�ı���ʽΪ ���������¶�ʱ��Kh�� ���������С�������䡱����
��2��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵΪ ��a��b�Ĺ�ϵΪ ������ڡ���С�ڡ������ڡ�����
��3����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ 0.01mol��L-1�������м����������ƣ�ʹ��Ũ��Ϊ0.9mol��L-1���������ֽ��������� �����ɳ�����ԭ���� ����KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
![]() =2.2��
=2.2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��Ϊ�˽�������β���Դ�������Ⱦ���йز������ü״����Һ��ʯ������Ϊ��������ȼ�ϡ���֪����25��10kPa�£�10g�״�ȼ������CO2��Һ̬ˮʱ����226.8kJ����д����ʾ�״�ȼ�յ��Ȼ�ѧ����ʽ��
��2�� ijЩ��ѧ��Ӧ������ʽ��ʾ��A + B C + D + H2O
��ش��������⣺
����A��D����������������C�ǼҼһ����ij����ر��ĵ�ζƷ֮һ��д���÷�Ӧ�����ӷ���ʽ ��
����AΪˮ������Ҫ�ɷ�֮һ��B���ճ�������л��������Ʒ����
д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
����C��D��Ϊ�����Ҷ���ʹ����ʯ��ˮ����ǣ���ֻ��
�� ����д�����Լ������ƣ��Ϳ��Լ���C��D�������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���人�и����������в��Ի�ѧ�Ծ� ���ͣ������
��12 �֣���Դ����������������ٵ��ش���⡣�״���δ����Ҫ����ɫ��Դ֮һ��
��l����֪���� 25 �桢101 kPa ��,1g �״�ȼ������ CO2��Һ̬ˮʱ���� 22��70kJ ����д���״�ȼ�յ��Ȼ�ѧ����ʽ ��
��2����CO2��H2�ϳɼ״��Ļ�ѧ����ʽΪ��CO2��g��+ 3H2
��g��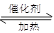 CH3OH��g��+H2O ��g ��.�������������������£�ʵ�����¶ȶԷ�Ӧ��Ӱ������ͼ��ʾ��ע��T1��T2������300 �棩
CH3OH��g��+H2O ��g ��.�������������������£�ʵ�����¶ȶԷ�Ӧ��Ӱ������ͼ��ʾ��ע��T1��T2������300 �棩
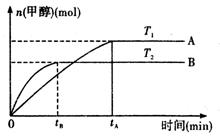
�ٺϳɼ״���Ӧ�ġ�H 0�����>������<����=�� ����
��ƽ�ⳣ���ı���ʽΪ: ���¶�ΪT2ʱ��ƽ�ⳣ�� �¶�ΪT1ʱ��ƽ�ⳣ�����>������<����=����
����T1�¶��£���1mol CO2�� 1 molH2����һ�ܱպ��������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊ�����������ڵ�ѹǿ����ʼѹǿ�ı�ֵΪ ��
��3�����ü״�ȼ�ϵ���������ͼ��ʾ��װ�á�
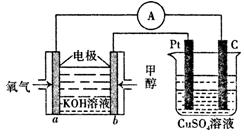
��װ���� Pt ��Ϊ ����д�� b���ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ൺ���и߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com