
ЫФДЈгаЗсИЛЕФЬьШЛЦјзЪдДЃЌвдЬьШЛЦјЮЊдСЯКЯГЩФђЫиЕФжївЊВНжшШчЯТЭМЫљЪО(ЭМжаФГаЉзЊЛЏВНжшМАЩњГЩЮяЮДСаГі)ЃК
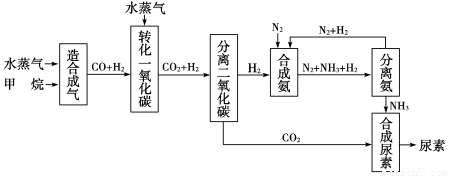
ЧыЬюаДЯТСаПеАзЃК
(1)вбжЊ0.5 molМзЭщгы0.5 molЫЎеєЦјдкt ЁцЁЂp kPaЪБЃЌЭъШЋЗДгІЩњГЩвЛбѕ
ЛЏЬМКЭЧтЦј(КЯГЩЦј)ЃЌЮќЪеСЫa kJШШСПЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪНЪЧЃК______________________ЁЃ
(2)дкКЯГЩАБЕФЪЕМЪЩњВњЙ§ГЬжаЃЌГЃВЩШЁЕФДыЪЉжЎвЛЪЧЃКНЋЩњГЩЕФАБДгЛьКЯЦјЬхжаМАЪБЗжРыГіРДЃЌВЂНЋЗжРыГіАБКѓЕФЕЊЦјКЭЧтЦјбЛЗРћгУЃЌЭЌЪБВЙГфЕЊЦјКЭЧтЦјЁЃЧыдЫгУЛЏбЇЗДгІЫйТЪКЭЛЏбЇЦНКтЕФЙлЕуЫЕУїВЩШЁИУДыЪЉЕФРэгЩЃК
________________________________________________________________ЁЃ
(3)ЕБМзЭщКЯГЩАБЦјЕФзЊЛЏТЪЮЊ75%ЪБЃЌвд5.60ЁС107 LМзЭщЮЊдСЯФмЙЛКЯГЩ________LАБЦјЁЃ(МйЩшЬхЛ§ОљдкБъзМзДПіЯТВтЖЈ)
(4)вбжЊФђЫиЕФНсЙЙМђЪНЮЊ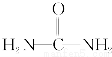 ЃЌЧыаДГіСНжжКЌгаЬМбѕЫЋМќЕФФђЫиЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК
ЃЌЧыаДГіСНжжКЌгаЬМбѕЫЋМќЕФФђЫиЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК
Ђй__________________ЃЛ Ђк_________________ЁЃ
(1)CH4(g)ЃЋH2O(g)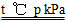 CO(g)ЃЋ3H2(g) ІЄHЃНЃЋ2a kJЁЄmolЃ1
CO(g)ЃЋ3H2(g) ІЄHЃНЃЋ2a kJЁЄmolЃ1
(2)діДѓЕЊЦјКЭЧтЦјЕФХЈЖШгаРћгкдіДѓЗДгІЫйТЪЃЛМѕаЁАБЦјЕФХЈЖШЁЂдіДѓЕЊЦјКЭЧтЦјЕФХЈЖШОљгаРћгкЦНКтЯђе§ЗДгІЗНЯђвЦЖЏ
(3)8.4ЁС107
(4)Ђй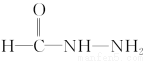 ЁЁЂкNH4NЃНCЃНO
ЁЁЂкNH4NЃНCЃНO
ЁОНтЮіЁП(1)0.5 mol CH4ЭъШЋЗДгІЮќЪеa kJШШСПЃЌдђ1 mol CH4ЭъШЋЗДгІЮќЪе2a kJШШСПЃЌШШЛЏбЇЗНГЬЪНЮЊCH4(g)ЃЋH2O(g)=CO(g)ЃЋ3H2(g)ЁЁІЄHЃНЃЋ2a kJЁЄmolЃ1ЁЃ
(2)ВЩгУбЛЗВйзїгаРћгкдіДѓЗДгІЮяХЈЖШЃЌдіДѓЗДгІЫйТЪЃЌЬсИпзЊЛЏТЪЃЛМѕЩйNH3ХЈЖШгаРћгкЦНКте§ЯђвЦЖЏЁЃ
(3)гЩЗДгІЛЏбЇЗНГЬЪНПЩЧѓзЊЛЏЙиЯЕ1CH4ЁЋ3H2ЁЋ2NH3ЃЌV(NH3)ЃН5.60ЁС107 LЁС75%ЁС2ЃН8.4ЁС107 LЁЃ
(4)ПЩЯШаДГі НсЙЙЃЌдйгыЦфгрЛљЭХСЌНгЁЃПЩаДГівьЙЙЬх
НсЙЙЃЌдйгыЦфгрЛљЭХСЌНгЁЃПЩаДГівьЙЙЬх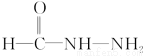 КЭNH4NЃНCЃНOЕФНсЙЙМђЪНЁЃ
КЭNH4NЃНCЃНOЕФНсЙЙМђЪНЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 3.1 ЮоЛњЗЧН№ЪєВФСЯСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЙигкЙЄвЕЩњВњЕФа№ЪіЃЌДэЮѓЕФЪЧ (ЁЁЁЁ)ЁЃ
AЃЎжЦЦеЭЈВЃСЇЕФжївЊдСЯЪЧДПМюЁЂЪЏЛвЪЏЁЂЪЏгЂ
BЃЎАБЪЧжЦзїЕЊЗЪЁЂЯѕЫсЁЂяЇбЮЕФживЊдСЯ
CЃЎНЋЖўбѕЛЏСђДпЛЏбѕЛЏЩњГЩШ§бѕЛЏСђКѓЃЌдкЮќЪеЫўФкгУЫЎЮќЪежЦЕУХЈСђЫс
DЃЎжЦдьЦеЭЈЫЎФрЕФжївЊдСЯЪЧ№ЄЭСЁЂЪЏЛвЪЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 2.1 ЛёШЁНрОЛЕФЫЎСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЛЧЛЏУК(ДњБэЪНNaR)ЪЧвЛжжбєРызгаЭНЛЛЛМСЃЌЫќФмЪЙгВЫЎжаЕФCa2ЃЋЁЂMg2ЃЋЭЈЙ§НЛЛЛГ§ШЅЖјШэЛЏЁЃЯждкКЃЫЎ(вбжЊКЃЫЎжаКЌNaЃЋЁЂCa2ЃЋЁЂMg2ЃЋЕШбєРызг)ЕФЕЛЏЗНЗЈЪЧЪЙКЃЫЎАДЫГађЭЈЙ§СНжжРызгНЛЛЛЪїжЌЃЌЦфСїГЬШчгвЭМЫљЪОЁЃ
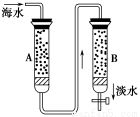
(1)ЯжгаЧтаЭбєРызгНЛЛЛЪїжЌКЭєЧаЭвѕРызгНЛЛЛЪїжЌЃЌдђ(ЬюДњБэЪН)AжљЪЧ________________ЃЌBжљЪЧ__________________ЁЃ
(2)АДЩЯЪіЫГађзАжљЕФРэгЩЪЧ_________________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 1.3 ДПМюЕФЩњВњСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЯТЭМБэЪОЕФЪЧКюЪЯжЦМюЗЈЙЄвеСїГЬЪОвтЭМЁЃ
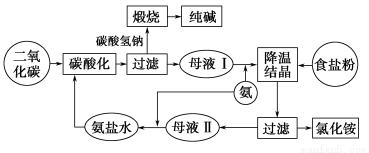
ЧыЛиД№ЃК
(1)ФИвКЂђжаЫљШмНтЕФжївЊЮяжЪЪЧ________(аДЮяжЪЕФЛЏбЇЪН)ЁЃФИвКЂђжаКЌгаЕЭХЈЖШЕФNa2CO3ЃЌжївЊдвђЪЧ__________________________
(2)ЯђФИвКЂђжаЭЈШыЦјЬхЪБвЊЯШЭЈАБЦјКѓЭЈЖўбѕЛЏЬМЦјЬхЃЌжївЊдвђЪЧ_______________________________________
(3)ФИвКЂёКЭФИвКЂђжаЖМЭЈШыNH3ЁЃЂйNH3ЕФжївЊРДдДЪЧ__________ЃЛЂкФИвКЂёжаЭЈШыNH3ЕФжївЊФПЕФЪЧ____________ЃЛЂлФИвКЂђжаЭЈШыNH3ЕФжївЊФПЕФЪЧ__________________ЁЃ
(4)ЙЄвеСїГЬЕФЁАьбЩеЁњДПМюЁБДІЃЌПЩЩшМЦ____________ЕФбЛЗЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 1.3 ДПМюЕФЩњВњСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ДПМюЪЧвЛжжживЊЕФЛЏЙЄдСЯЁЃФПЧАжЦМюЙЄвЕжївЊгаЁААБМюЗЈЁБКЭЁАСЊКЯжЦМюЗЈЁБСНжжЙЄвеЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ (ЁЁЁЁ)ЁЃ
AЃЎЁАСЊКЯжЦМюЗЈЁБКЭЁААБМюЗЈЁБЕФЛЏбЇЗДгІдРэжаЖМгаЯТСаЛЏбЇЗДгІ
NH3ЃЋCO2ЃЋNaClЃЋH2O=NaHCO3Ё§ЃЋNH4Cl
2NaHCO3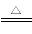 Na2CO3ЃЋH2OЁќЃЋCO2Ёќ
Na2CO3ЃЋH2OЁќЃЋCO2Ёќ
BЃЎЁАСЊКЯжЦМюЗЈЁБЩњВњжагаАБЕФбЛЗРћгУЙЄве
CЃЎЁААБМюЗЈЁБЩњВњжагаАБЕФбЛЗРћгУЙЄве
DЃЎЁАСЊКЯжЦМюЗЈЁБКЭЁААБМюЗЈЁБЖМгавЛЖЈЕФОжЯоад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 1.2 ШЫЙЄЙЬЕЊКЯГЩАБСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
КЯГЩАБЙЄвЕжаЃЌдСЯЦј(N2ЁЂH2ЁЂЛьгаЩйСПCOЁЂNH3)дкНјШыКЯГЩЫўжЎЧАЃЌГЃгУДзЫсЖўАБКЯЭ(Ђё)ШмвКРДЮќЪеCOЃЌЦфЗДгІЮЊCH3COO[Cu(NH3)2]ЃЋCOЃЋNH3
CH3COO[Cu(NH3)3]ЁЄCO(е§ЗДгІЮЊЗХШШЗДгІ)ЁЃ
(1)БиаыГ§ШЅCOЕФдвђЪЧ___________________________ЁЃ
(2)ДзЫсЖўАБКЯЭ(Ђё)ШмвКЮќЪедСЯЦјжаCOЕФЪЪвЫЬѕМўЪЧ______________________ЁЃ
(3)ЮќЪеCOКѓЕФДзЫсЭ(Ђё)АБШмвКОЪЪЕБДІРэгжПЩдйЩњЃЌЛжИДЦфЮќЪеCOЕФФмСІЖјбЛЗЪЙгУЃЌЦфдйЩњЕФЬѕМўЪЧ______________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаоЖў 1.1 ЛЏЙЄЩњВњжаЕФЮЪЬтСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
СђЫсЙЄвЕдкЙњУёОМУжаеМгаМЋЦфживЊЕФЕиЮЛЁЃ
(1)СђЫсЕФзюДѓЯћЗбЧўЕРЪЧЛЏЗЪЙЄвЕЃЌгУСђЫсжЦдьЕФГЃМћЛЏЗЪга________(ШЮаДвЛжж)ЁЃ
(2)СђЫсЩњВњжаЃЌИљОнЛЏбЇЦНКтдРэРДШЗЖЈЕФЬѕМўЛђДыЪЉга________(ЬюаДађКХ)ЁЃ
AЃЎПѓЪЏМгШыЗаЬкТЏжЎЧАЯШЗлЫщ
BЃЎЪЙгУV2O5зїДпЛЏМС
CЃЎзЊЛЏЦїжаЪЙгУЪЪвЫЕФЮТЖШ
DЃЎОЛЛЏКѓЕФТЏЦјжавЊгаЙ§СПЕФПеЦј
EЃЎДпЛЏбѕЛЏдкГЃбЙЯТНјаа
FЃЎЮќЪеЫўжагУ98.3%ЕФХЈСђЫсЮќЪеSO3
(3)дкСђЫсЙЄвЕжаЃЌЭЈЙ§ЯТСаЗДгІЪЙЖўбѕЛЏСђзЊЛЏЮЊШ§бѕЛЏСђЃК2SO2(g)ЃЋ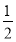 O2(g)
O2(g)  2SO3(g) ІЄHЃНЃ98.3 kJЁЄmolЃ1ЁЃдкЪЕМЪЙЄвЕЩњВњжаЃЌГЃВЩгУЁАЖўзЊЖўЮќЗЈЁБЃЌМДНЋЕквЛДЮзЊЛЏЩњГЩЕФSO2ЗжРыКѓЃЌНЋЮДзЊЛЏЕФSO2НјааЖўДЮзЊЛЏЃЌМйШєСНДЮSO2ЕФзЊЛЏТЪОљЮЊ95%ЃЌдђзюжеSO2ЕФзЊЛЏТЪЮЊ________ЁЃ
2SO3(g) ІЄHЃНЃ98.3 kJЁЄmolЃ1ЁЃдкЪЕМЪЙЄвЕЩњВњжаЃЌГЃВЩгУЁАЖўзЊЖўЮќЗЈЁБЃЌМДНЋЕквЛДЮзЊЛЏЩњГЩЕФSO2ЗжРыКѓЃЌНЋЮДзЊЛЏЕФSO2НјааЖўДЮзЊЛЏЃЌМйШєСНДЮSO2ЕФзЊЛЏТЪОљЮЊ95%ЃЌдђзюжеSO2ЕФзЊЛЏТЪЮЊ________ЁЃ
(4)СђЫсЕФЙЄвЕжЦЗЈЙ§ГЬЩцМАШ§ИіжївЊЕФЛЏбЇЗДгІМАЯргІЕФЩшБИ(ЗаЬкТЏЁЂзЊЛЏЦїЁЂЮќЪеЫў)ЁЃ
ЂйШ§ИіЩшБИЗжБ№ЪЙЗДгІЮяжЎМфЛђРфШШЦјЬхжЎМфНјааЁАЖдСїЁБЁЃЧыМђЕЅУшЪіЮќЪеЫўжаЗДгІЮяжЎМфЪЧдѕбљЖдСїЕФЁЃ______________________________ЁЃ
ЂкЙЄвЕЩњВњжаГЃгУАБЃЫсЗЈНјааЮВЦјЭбСђЃЌвдДяЕНЯћГ§ЮлШОЁЂЗЯЮяРћгУЕФФПЕФЁЃгУЛЏбЇЗНГЬЪНБэЪОЦфЗДгІдРэЁЃ(жЛаДГі2ИіЗНГЬЪНМДПЩ)
________________________________________________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаовЛ ФЃПщзлКЯМьВтСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСагаЙиВФСЯЕФЫЕЗЈжаВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉЁЃ
A.ДЋЭГЕФЮоЛњЗЧН№ЪєВФСЯЫфгаВЛЩйгХЕуЃЌЕЋжЪДрЃЌОВЛЦ№ШШГхЛї
B.аТаЭЮоЛњЗЧН№ЪєВФСЯЫфШЛПЫЗўСЫДЋЭГЮоЛњЗЧН№ЪєВФСЯЕФШБЕуЃЌЕЋЧПЖШБШНЯВю
C.ИпЮТНсЙЙВФСЯОпгаФмФЭИпЮТЃЌВЛХТбѕЛЏЃЌФЭЫсМюИЏЪДЃЌУмЖШаЁЕШгХЕу
D.аТаЭЮоЛњЗЧН№ЪєВФСЯЬиаджЎвЛЪЧОпгаЙтбЇЬиад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўЛЏбЇШЫНЬАцбЁаовЛ 4.2 АЎЛЄЫЎзЪдДСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
НќФъРДГрГБдкЮвЙњЪБгаЗЂЩњЃЌЕБГрГБЗЂЩњЪБЃЌКЃЫЎжаЕФФГаЉЮЂаЁИЁгЮЩњЮяДѓСПЗБжГЃЌЪЙЫЎЬхГЪЯжКьЁЂзЯЕШбеЩЋЃЌВЂЖдЩњЮядьГЩЮЃКІЁЃЯТСаЫЕЗЈжаВЛе§ШЗЕФЪЧ( )ЁЃ
AЃЎГрГБЪЧЫЎЬхИЛгЊбјЛЏЕФНсЙћ
BЃЎКЌСзЯДЕгМСЕФЙуЗКЪЙгУгыХХЗХЪЧГрГБЗЂЩњЕФжївЊдвђ
CЃЎдкЗтБеЕФКЃЭхИќШнвзЗЂЩњГрГБ
DЃЎГрГБЯжЯѓЮЊгуРрЕШЬсЙЉСЫДѓСПЪГЮяЃЌгаРћгкЫќУЧЕФДѓСПЗБжГгыЩњГЄ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com