
��пƬ��ϡ���ᷴӦ��ʵ������¼���
���� | ����Zn����/g | ����ϡ��������/g | ����ZnSO 4 ����/g |
1 | 2 | 60 | 5 |
2 | 4 | 60 |
|
3 | 6 | 60 | 15 |
4 | 8 | 60 | 17 |
5 | 10 | 60 |
|
6 | 12 | 60 | 17 |
��1����2��5�����ZnSO4 ������������______________g.
��2����ͼ�л���ZnSO4 ��Zn������������ϵ������.

��3��(10+m)gп��60gϡ�����ַ�Ӧ��ʣ����������Ϊ______________g.
��4��ϡ�������������������______________.
��1��10g��17g
��2����ͼ����û�б������ֵ����.��������п���������Լ�������п������Ϊ6.9g��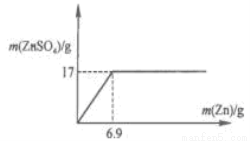
��3����3.1g+m��g �� ��4��17.2%
��������
������������ݱ�����ǰ4�ε����ݿ��Կ�����������������䣬Zn���������ӣ�������ZnSO4 ����Ҳ�����ӣ�˵�����������ԭ���2�ε������ǵ�һ�ε�2�������Բ�����ZnSO4����Ҳ�ǵ�һ�ε�2����������2��5g=10g�����Ĵβ���ZnSO4������17g,��6��Zn�������ӣ���������ZnSO4�������䣬˵�����ᷴӦ��ȫ�����Ե�5�μ���Zn������10gʱ������ZnSO4����Ҳ��17g����2��65gZn��ȫ��Ӧ�����161g ZnSO4�������17g ZnSO4���ĵ�Zn�������ǣ���17��65g����161=6.9g����ZnSO4 ��Zn������������ϵ��������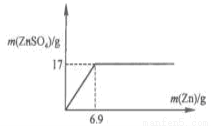 ��
��
��3��(10+m)gп��60gϡ�����ַ�Ӧ�����ZnSO4������17g������Zn��������6.9gZn���������ӣ�Ҳ���ᷢ����Ӧ������ʣ����������Ϊ(10+m)g��6.9g=��3.1g+m��g����4�����������غ㶨�ɿ�֪��17g ZnSO4�к��е�SO42-��������17g-6.9g=10.1g�������ĵ�����������ǣ�98��96����10.1g=10.3g,����ϡ�������������������(10.3g��60g)��100%=17.2%��
���㣺����ʵ�����ݵĴ�����ͼ��ʾ�����ʵ�������ϵ����Һ�����������ļ����֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶���ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʣ������� ��ʳ��ˮ ���ۻ����Ȼ��� ��Һ̬�Ȼ��� ��ͭ �ް�ˮ ��SO3 ����ᣬ���п��Ե��粢������ǿ����ʵ��ǣ� ��
A��ֻ�Т� B���٢ڢۢޢ� C���ܢ� D��ȫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶�9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ʳ���������ʲ��������ж�����
A���������谷���Ƶ��̷�
B�����д����������ƣ�nano 2 ���Ļ���
C����̼�ᱵ��x�����ӵġ����͡�
D���ӵ��Σ�������ص�ʳ�Σ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�������
13gп��100gϡ����ǡ����ȫ��Ӧ����������Һ�����ʵ���������.(��������ȷ��0.1%)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ������
���ܽ����______________�仯Ӱ�첻��Ĺ�������һ����______________�����õ��������ʣ�����Ӻ�ˮ����ȡ______________�����ܽ����______________�仯Ӱ���൱��Ĺ�������һ����______________�ķ����õ�����.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ� ��
A��ȼ�ն����������
B��������ȼ�ױ���Ĺ����������Ͻ����������װ
C��ú���б����ȡͨ�磬�Ͻ��̻�
D����ȼ���¶ȴﵽ�Ż�㼴��ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ʱ����ȼ���������ˮ����Ҫ�����ǣ� ��
A���������� B�������¶� C��ʹˮ�ֽ� D��ʹˮ���ˮ�����Իӷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪���ӱ������������ϵ�һ���¿���һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��3�֣�����˵����ȷ���ǣ� ��
A�� 1mol�κ�������ռ�����ԼΪ22.4L
B�� 1molH2O�ڱ�״�������Ϊ22.4L
C�� 1mol�������Ϊ22.4L����һ���DZ�״��
D�� ����Ħ�������һ����22.4L/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�걱���и߶��ϵ�һ��������⻯ѧ�Ծ��������棩 ���ͣ������
��CaCO3= CaO + CO2 ��H=+177.7kJ��mol-1
��C(s) + H2O(g) = CO(g) + H2(g) ��H=131.3kJ��mol-1
��1/2 H2SO4(aq) + NaOH(aq) =1/2Na2SO4(aq) + H2O(l) ��H= ��57.3kJ
��C(s) + O2(g) = CO2(g) ��H=��393.5kJ��mol-1
��CO(g) + 1/2O2(g) = CO2(g) ��H=��283kJ��mol-1
��HNO3(aq) + NaOH(aq) = NaNO3(aq) + H2O(l) ��H=��57.3kJ��mol-1
��1�������Ȼ�ѧ����ʽ�У�����ȷ���� ��
��2��������Ӧ�У���ʾȼ���ȵ��Ȼ�ѧ����ʽ�� ����ʾ�к��ȵ��Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com