
ЎҫМвДҝЎҝҪ«0.8 mol I2(g)әН1.2 mol H2(g)ЦГУЪДі1LГЬұХИЭЖчЦРЈ¬ФЪТ»¶ЁОВ¶ИПВ·ўЙъ·ҙУҰЈәI2(g)Ј«H2(g) ![]() 2HI(g)ІўҙпөҪЖҪәвЎЈHIөДМе»э·ЦКэЛжКұјдөДұд»ҜИзұнёсЛщКҫЈә
2HI(g)ІўҙпөҪЖҪәвЎЈHIөДМе»э·ЦКэЛжКұјдөДұд»ҜИзұнёсЛщКҫЈә
HIМе»э·ЦКэ | 1min | 2min | 3min | 4min | 5min | 6min | 7min[ |
МхјюI | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
МхјюII | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
ЈЁ1Ј©ФЪМхјюIөҪҙпЖҪәвКұЈ¬јЖЛгёГ·ҙУҰөДЖҪәвіЈКэKЈ¬ТӘЗуБРіцјЖЛг№эіМЎЈ
ЈЁ2Ј©ФЪМхјюIҙУҝӘКј·ҙУҰөҪөҪҙпЖҪәвКұЈ¬H2өД·ҙУҰЛЩВКОӘ____________ЎЈ
ЈЁ3Ј©ОӘҙпөҪМхјюIIөДКэҫЭЈ¬¶ФУЪ·ҙУҰМеПөҝЙДЬёДұдөДІЩЧчКЗ_______________ЎЈ
ЈЁ4Ј©ёГ·ҙУҰөДЎчH__________0ЈЁМо">"Ј¬"<"»т"="Ј©
ЈЁ5Ј©ФЪМхјюIПВҙпөҪЖҪәвәуЈ¬ФЪ7minКұҪ«ИЭЖчМе»эС№ЛхОӘФӯАҙөДТ»°лЎЈЗлФЪНјЦР»ӯіцc(HI)ЛжКұјдұд»ҜөДЗъПЯЎЈ
Ўҫҙр°ёЎҝЈЁ1Ј©ЙиI2ПыәДЕЁ¶ИОӘx
I2(g) + H2(g) ![]() 2HI(g)
2HI(g)
ЖрКјЕЁ¶ИЈЁmol/LЈ©Јә0.8 1.2 0
ЧӘ»ҜЕЁ¶ИЈЁmol/LЈ©Јәx x 2x
ЖҪәвЕЁ¶ИЈЁmol/LЈ©Јә0.8-x 1.2-x 2x
HIөДМе»э·ЦКэОӘ60%Ј¬ФтЈә2x/2=60%Ј¬x=0.6 mol/L
K=c2 (HI) /[c(H2)ЎӨc(I2)]=1.22/(0.2ЎБ0.6)=12Ј»
ЈЁ2Ј©0.12 mol/(LЎӨmin)ЈЁ3Ј©ҪөөНОВ¶ИЈ»ЈЁ4Ј©<Ј»
ЈЁ5Ј©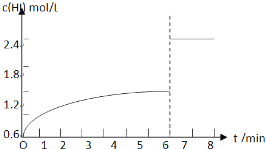
ЎҫҪвОцЎҝ
КФМв·ЦОцЈәЈЁ1Ј©УЙұнЦРКэҫЭҝЙЦӘЈ¬МхјюIПВ5minКұҙҰУЪЖҪәвЧҙМ¬Ј¬ЙиI2ПыәДЕЁ¶ИОӘxmol/LЈ¬ФтЈә
I2ЈЁgЈ©+H2ЈЁgЈ©![]() 2HIЈЁgЈ©
2HIЈЁgЈ©
ЖрКјЕЁ¶ИЈЁmol/LЈ©Јә0.8 1.2 0
ЧӘ»ҜЕЁ¶ИЈЁmol/LЈ©Јәx x 2x
ЖҪәвЕЁ¶ИЈЁmol/LЈ©Јә0.8-x 1.2-x 2x
HIөДМе»э·ЦКэОӘ60%Ј¬ФтЈә![]() =60%Ј¬№Кx=0.6Ј¬ЖҪәвіЈКэK= c2 (HI) /[c(H2)ЎӨc(I2)]=1.22/(0.2ЎБ0.6)=12Ј¬№Кҙр°ёОӘЈә12Ј»
=60%Ј¬№Кx=0.6Ј¬ЖҪәвіЈКэK= c2 (HI) /[c(H2)ЎӨc(I2)]=1.22/(0.2ЎБ0.6)=12Ј¬№Кҙр°ёОӘЈә12Ј»
ЈЁ2Ј©ФЪМхјюIҙУҝӘКј·ҙУҰөҪөҪҙпЖҪәвКұЈ¬H2өД·ҙУҰЛЩВКОӘ![]() =0.12 mol/ЈЁLminЈ©Ј¬№Кҙр°ёОӘЈә0.12 mol/ЈЁLminЈ©Ј»
=0.12 mol/ЈЁLminЈ©Ј¬№Кҙр°ёОӘЈә0.12 mol/ЈЁLminЈ©Ј»
ЈЁ3Ј©ПаН¬КұјдДЪHIөДМе»э·ЦКэјхРЎЈ¬ЛөГч·ҙУҰЛЩВКјхВэЈ¬ЖҪәвКұHIМе»э·ЦКэҙуУЪМхјюIКұЈ¬№КёДұдМхјюЖҪәвХэПтТЖ¶ҜЈ¬УЙУЪС№ЗҝЎўҙЯ»ҜјБІ»У°ПмЖҪәвТЖ¶ҜЈ¬ҝЙДЬКЗҪөөНОВ¶ИЈ¬№Кҙр°ёОӘЈәҪөөНОВ¶ИЈ»
ЈЁ4Ј©ҪөөНОВ¶ИЖҪәвКұХэПтТЖ¶ҜЈ¬ЛөГчХэ·ҙУҰОӘ·ЕИИ·ҙУҰЈ¬јҙЎчHЈј0Ј¬№Кҙр°ёОӘЈәЈјЈ»
ЈЁ5Ј©ФЪМхјюIПВҙпөҪЖҪәвәуЈ¬HIөДЕЁ¶ИОӘ1.2mol/LЈ¬ФЪ7minКұҪ«ИЭЖчМе»эС№ЛхОӘФӯАҙөДТ»°лЈ¬С№ЗҝФцҙуЈ¬ЖҪәвІ»ТЖ¶ҜЈ¬HIөДЕЁ¶ИұдОӘФӯЖҪәвөД2ұ¶Ј¬јҙHIЕЁ¶ИұдОӘ2.4mol/LЈ¬cЈЁHIЈ©ЛжКұјдұд»ҜөДЗъПЯОӘЈә



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝAЎўBЎўCЎўDЎўEКЗФӯЧУРтКэТАҙОФцҙуөДОеЦЦ¶МЦЬЖЪЦчЧеФӘЛШЈ¬ЖдЦРAөДФӯЧУРтКэКЗBәНDФӯЧУРтКэЦ®әНөД1/4Ј¬CФӘЛШөДЧоёЯјЫСх»ҜОпөДЛ®»ҜОпКЗТ»ЦЦЦРЗҝјоЈ¬јЧәНұыКЗDФӘЛШөДБҪЦЦіЈјыСх»ҜОпЈ¬ТТәН¶ЎКЗBФӘЛШөДБҪЦЦіЈјыН¬ЛШТмРОМеЈ¬0.005mol/LОмИЬТәөДpH=2Ј¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫ(Іҝ·Ц·ҙУҰОпКЎВФ)Ј¬ПВБРРрКцХэИ·өДКЗЈЁ Ј©
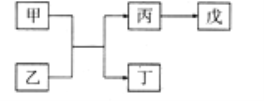
A. CЎўDБҪФӘЛШРОіЙ»ҜәПОпКф№ІјЫ»ҜәПОп
B. CЎўDөДјтөҘАлЧУҫщДЬҙЩҪшЛ®өДөзАл
C. AЎўD·ЦұрУлBФӘЛШРОіЙөД»ҜәПОп¶јКЗҙуЖшОЫИҫОп
D. EөДСх»ҜОпЛ®»ҜОпөДЛбРФҙуУЪDөДСх»ҜОпЛ®»ҜОпөДЛбРФ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРОпЦКІ»КфУЪәПҪрөДКЗЈЁ Ј©
A. І»РвёЦ B. ЗаНӯ C. УІВБ D. КҜУў
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝИзНјЛщКҫИэёцЙХЖҝЦР·ЦұрЧ°Илә¬·УМӘөД0.01mol/LCH3COONaИЬТәЈ¬Іў·Цұр·ЕЦГФЪКўУРЛ®өДЙХұӯЦРЈ¬И»әуПтЙХұӯўЩЦРјУИлЙъКҜ»ТЈ¬ПтЙХұӯўЫЦРјУИлNH4NO3ҫ§МеЈ¬ЙХұӯўЪЦРІ»јУИОәООпЦКЈ®ФтПВБРРрКцХэИ·өДКЗ
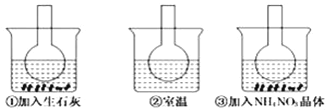
A. ўЩЛөГчЛ®Ҫв·ҙУҰОӘ·ЕИИ·ҙУҰ B. ўЫЛөГчЛ®Ҫв·ҙУҰОӘОьИИ·ҙУҰ
C. ўЩЦРИЬТәәмЙ«ұдЗі D. ўЫЦРИЬТәәмЙ«ұдЙо
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРРрКцХэИ·өДКЗ
A. Гч·ҜәНЖҜ°Ч·ЫіЈУГУЪЧФАҙЛ®өДҫ»»ҜәНПы¶ҫЈ¬БҪХЯФӯАнПаН¬
B. іЈОВПВЈ¬Н¬ЕЁ¶ИөДNa2SУлNaHSИЬТәПаұИЈ¬Na2SИЬТәөДpHРЎ
C. өИОпЦКөДБҝЕЁ¶ИөДNH4ClИЬТәәНNH4HSO4ИЬТәЈ¬әуХЯөДc(NH4Ј«)ҙу
D. FeCl3УлKSCN·ҙУҰҙпөҪЖҪәвКұЈ¬јУИлKClИЬТәЈ¬ФтИЬТәСХЙ«ұдЙо
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҪбәПНјЕР¶ПЈ¬ПВБРРрКцХэИ·өДКЗ
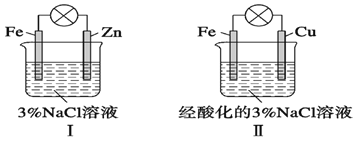
A. ўсәНўтЦРХэј«ҫщұ»ұЈ»Ө
B. ўсәНўтЦРёәј«·ҙУҰҫщКЗFeЈӯ2eЈӯ=Fe2Ј«
C. ўсәНўтЦРХэј«·ҙУҰҫщКЗO2Ј«2H2OЈ«4eЈӯ=4OHЈӯ
D. ўсәНўтЦР·ЦұрјУИлЙЩБҝK3[Fe(CN)6]ИЬТәЈ¬ҫщУРА¶Й«іБөн
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПЦУРИэЧйКөСйЈәўЩіэИҘ»мФЪЦІОпУНЦРөДЛ®Ј»ўЪ¶ФөвЛ®ЦРөДөвҪшРРЕЁЛхЈ»ўЫ¶Ф30%өДҫЖҫ«ИЬТәЦРөДҫЖҫ«ҪшРРМбҙҝЎЈТФЙПКөСйІЙУГөДХэИ··Ҫ·ЁТАҙОКЗЈЁ Ј©
A.·ЦТәЎўЭНИЎЎўХфБуB.ЭНИЎЎўХфБуЎў·ЦТә
C.·ЦТәЎўХфБуЎўЭНИЎD.ХфБуЎўЭНИЎЎў·ЦТә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЈЁ1Ј©ұкҝцПВ5.6LөДCO2ЦКБҝОӘ _____g,ЖдЦРә¬УР______ёц·ЦЧУЈ¬ә¬УР_____ёцФӯЧУЈ»
ЈЁ2Ј©ЦКБҝҫщОӘm gөД HClЎўNH3ЎўCO2ЎўO2ЛДЦЦЖшМеЈ¬Лщә¬·ЦЧУКэДҝЧоЙЩөДКЗ_________Ј¬Ме»эЧоҙуөДКЗ_____Ј¬ГЬ¶ИЧоРЎөДКЗ_______ЈЁФЪПаН¬ОВ¶ИәНС№ЗҝМхјюПВЈ©ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝәЈЛ®ХјөШЗтЧЬҙўЛ®БҝөД97.2%ЎЈИф°СәЈЛ®өӯ»ҜәН»Ҝ№ӨЙъІъҪбәПЖрАҙЈ¬јИҝЙТФҪвҫцөӯЛ®ЧКФҙИұ·ҰөДОКМвЈ¬УЦҝЙТФід·ЦАыУГәЈСуЧКФҙЎЈ
ЈЁ1Ј©ДҝЗ°№ъјКЙПКөУГөДЎ°әЈЛ®өӯ»ҜЎұЦчТӘјјКхЦ®Т»КЗХфБу·ЁЎЈХфБу·ЁКЗҪ«әЈЛ®ұдіЙХфЖыЈ¬ХфЖыҫӯ№эАдИҙ¶шөГёЯҙҝ¶ИөӯЛ®Ј¬УЙҙЛҝЙЕР¶ПХфБу·ЁКЗ__________ЈЁМоОпАнұд»ҜЎў»ҜС§ұд»ҜЈ©Ј»
ЈЁ2Ј©КөСйКТУГMnO2әНЕЁСОЛбОӘФӯБПЦЖұёВИЖшЈ¬ёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ________________________________Ј¬ЖдЦРСх»ҜјБКЗ_______»№ФӯјБКЗ________ЎЈОІЖшҙҰАнөДАлЧУ·ҪіМКҪОӘ______________________________ЎЈ
ЈЁ3Ј©№ӨТөЙПЦЖұёЖҜ°Ч·ЫөД»ҜС§·ҪіМКҪ___________________________________ЎЈ
ЈЁ4Ј©Ҫ«КөСйКТЦЖөДВИЖшЕдіЙРВЦЖөДВИЛ®ә¬УР¶аЦЦОўБЈЈ¬КФУГ·ҪіМКҪ»т»ҜС§КҪ»ШҙрПВБРОКМвЈә
Ҫ«ВИЛ®өОјУЧПЙ«КҜИпИЬТәЦРЈ¬ПИұдәмәуНКЙ«______________________________ЈЁ»ҜС§·ҪіМКҪЈ©ЖдЦРЈ¬ұдәмәуНКЙ«КЗТтОӘУР_________ЈЁ»ҜС§КҪЈ©ЙъіЙҫЯУРЖҜ°ЧРФЈ»
Ҫ«ВИЛ®өОИлПхЛбТшИЬТәЦРЈ¬УР°ЧЙ«іБөнЙъіЙ__________________________ЎЈЈЁАлЧУ·ҪіМКҪЈ©
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com