
 ��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺| ��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� |
�¶Ȳ� ��t/�� | |||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | 27.3 | ||
| 2 | 25 | 25 | 27.4 | ||
| 3 | 25 | 25 | 28.6 | ||
| Q |
| n(H2O) |
| (27.3��-25��)+(27.4��-25��) |
| 2 |
| -Q |
| n(H2O) |
| -0.9823kJ |
| 0.025mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
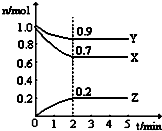 ij�¶�ʱ����һ��2L���ܱ������У�H2��N2��NH3�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ��ش��������⣺
ij�¶�ʱ����һ��2L���ܱ������У�H2��N2��NH3�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣺
ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ײ��ϵı�������ռ�������ı��������������������������ʵ�ԭ����ij�����������ӷֲ���������þ�������ṹ��ͼ��ʾ���������������ķ���ʽΪ���������� þԭ�ӡ�λ�ڶ���������������ģ���ԭ�ӡ�λ���ڲ� ��
���ײ��ϵı�������ռ�������ı��������������������������ʵ�ԭ����ij�����������ӷֲ���������þ�������ṹ��ͼ��ʾ���������������ķ���ʽΪ���������� þԭ�ӡ�λ�ڶ���������������ģ���ԭ�ӡ�λ���ڲ� ��| A��MgB |
| B��Mg3B6 |
| C��Mg5B12 |
| D��Mg14B6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��C2H6 |
| B��C2H2 |
| C��C2H4 |
| D��C3H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������л�������ƽ����Է���������С |
| B������Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ� |
| C������Ӧ���ʺ��淴Ӧ���ʶ���С��C�İٷֺ������� |
| D����������ܶȵı仯�����������Ϊ�жϷ�Ӧ�Ƿ��ٴδ�ƽ������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com