
 4NO+6H2O���÷�Ӧ�Ƿ��ȷ�Ӧ���¶�̫�߿���ʹ�����Ļ��Խ��ͣ�
4NO+6H2O���÷�Ӧ�Ƿ��ȷ�Ӧ���¶�̫�߿���ʹ�����Ļ��Խ��ͣ� 4NO+6H2O���÷�Ӧ�Ƿ��ȷ�Ӧ���¶�̫�߿���ʹ�����Ļ��Խ��ͣ�
4NO+6H2O���÷�Ӧ�Ƿ��ȷ�Ӧ���¶�̫�߿���ʹ�����Ļ��Խ��ͣ� Cr2O3+N2��+4H2O���ʴ�Ϊ����NH4��2Cr2O7
Cr2O3+N2��+4H2O���ʴ�Ϊ����NH4��2Cr2O7  Cr2O3+N2��+4H2O��
Cr2O3+N2��+4H2O��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(��)�¹��˹�����1905�귢���ĺϳɰ���Ӧԭ��Ϊ:N2(g)+3H2(g) ![]() 2NH3(g);��֪298 Kʱ,��H=-92.4 kJ��mol-1,��S=-198.2 J��mol-1��K-1.�Իش���������:
2NH3(g);��֪298 Kʱ,��H=-92.4 kJ��mol-1,��S=-198.2 J��mol-1��K-1.�Իش���������:
(1)���������Ӧ���ʱ���ر�������298 K�ºϳɰ���Ӧ���Է�����(�г���ʽ����)___________________.��Ũ����(Qc) __________________��ѧƽ�ⳣ��(Kc)(����ڡ������ڡ���С�ڡ���ʱ����Ӧ���ҽ���.
(2)��ʵ�ʹ�ҵ�ϳɰ������в�ȡ�Ĵ�ʩ��__________________ (�����).
A.���ýϵ�ѹǿ
B.����800 K���ҵĸ���
C.������ý������
D.�����ɵİ�Һ������ʱ����ϵ�з������,N2��H2ѭ�����ϳ����в�����N2��H2
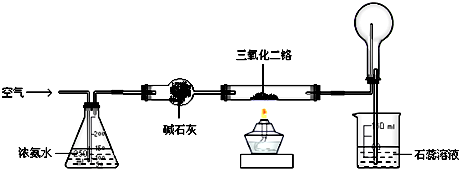
(3)����ͼ��ʾ��ʵ����ģ�ҵ���ϳɰ��ļ���װ��,���������а������ɵķ���
___________________________________________________________________________
___________________________________________________________________________.

(��)������һ����Ҫ�Ļ���ԭ��,��ҵ��ͨ�����ð���������ȡ.ijУ��ѧ��ȤС���ͬѧ���������ͼ��ʾװ��������ѧʵ���ҳ����Լ���ȡNH3,���Կ���,NH3Ϊԭ��ģ�ҵ��HNO3(����������Ϊ����,���ȼ��г�װ��δ����):

�ش���������:
(1)ʵ��ʱ,A,C��װ�þ������,Ӧ�ȼ���______________װ��,ԭ����___________________;
(2)Dװ����Ӧ����������______________,�����ʵ���Ҫ������______________;
(3)Eװ�õ�������______________,F,Gװ���е����ʷֱ���______________��______________;
(4)��������ͼ�к�ɫ���߿��ڵ�װ�õ�ȥ��ͨ�����ĵ���B,��Cװ���е�˫����Ƥ�����ɵ�����Ƥ��,������ͼʾ�ķ������һ�����ʵ�鷽��ͬ������������ȡ(������ͼ��ʾ�ķ����л���װ��ͼ��ע������ҩƷ������).

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡʮ��У����3�µ�һ��������ѧ�Ծ��������棩 ���ͣ������
��ҵ���������Ҫ��ӦΪ��4NH3��g��+5O2��g�� 4NO��g��+6 H2O��g����H��
4NO��g��+6 H2O��g����H��
��1����֪������ȼ����Ϊ285��8 kJ/mol��
N2��g��+3H2��g��=2NH3��g��? ��H=��92��4 kJ/mol;
H2O��1��=H2O��g����H=+44��0 kJ/mol;
N2��g��+O2��g��=2NO��g����H=+180��6 kJ/mol��
��������ҵ���������Ҫ��Ӧ����H=???????????????????????? ��
��2�����ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���

����Ӧ�ڵ�2 min����4 minʱ��O2��ƽ����Ӧ����Ϊ?????????????????? ��
����Ӧ�ڵ�6 minʱ�ı����������ı������������????????? ������ţ���
A��ʹ�ô���????? B�������¶�? C����Сѹǿ????? D������O2��Ũ��
������˵������˵��4NH3��g��+5O2��g�� 4NO��g��+6 H2 O��g���ﵽƽ��״̬����??????? ������ţ���
4NO��g��+6 H2 O��g���ﵽƽ��״̬����??????? ������ţ���
A����λʱ��������n mol NO����ʱ������n mol NH3
B������һ������������ƽ����Է����������ٱ仯
C���ٷֺ���w��NH3��=w��NO��
D����Ӧ����v��NH3����u��O2����v��NO����v��H2O��=4��5��4��6
E�����ں��º�ѹ���ݻ��ɱ�������з�Ӧ�����������ܶȲ��ٱ仯
��3��ij�о�����װ��CH3OH��O2ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��

���õ�ع���ʱ��b��ͨ�������Ϊ____????? ��
���õ�������ĵ缫��ӦʽΪ��???????????????????????? ��
���Դ˵������Դ����ʵ������ģ������Ʒ�������ۻ���������װ����ͼ��ʾ���Ĺ����У�������Һ����Dz������ݲ�������ԭ�������?????????????????????????????????? ������ص����ӷ���ʽ��ʾ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com