
���� ��1���ٸ������ƹ��������ʵ����ʵ�������������Ҫ12mol/L��Ũ����������
�۸���������Һ���ȷ������ƿ���
����ʱ��ֱ�Ӽ�ˮ������ý�ͷ�ιܶ��ݣ�
��2�����ݲ���������c=$\frac{n}{V}$��Ӱ��������ƹ����в�������
��� ��1����ʵ��������0.6mol/L��HCl��Һ250mL�����ƹ�����HCl�����ʵ������䣬����Ҫ12mol/L��Ũ��������Ϊ��$\frac{0.6mol/L��0.25L}{12mol/L}$=0.125L=12.5mL��
�ʴ�Ϊ��12.5��
������250mL��Һ��Ҫѡ��250mL����ƿ��������Һ��ȴ���ز�����ע��250mL����ƿ�У�
�ʴ�Ϊ��250��
������ˮע�뵽����ƿ�У�Һ����̶���1-2cmʱ�����ý�ͷ�ιܼ�����ˮʹ��Һ�����ʹ���̶������У�
�ʴ�Ϊ����ͷ�ιܣ�
��2����δϴ���ձ��Ͳ��������������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ڶ���ʱ�����ӿ̶��߶��������Ƶ���Һ���ڿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
������Һʱ��������ƿ������ˮϴ����δ�����ɱ���������Һ�������ʺ���Һ�����������Ӱ�죬��Ӱ�����ƽ����
�ʴ�Ϊ����Ӱ�죮
���� ���⿼������Һ���Ƶķ���������������Ŀ�Ѷ��еȣ���ȷ���Ʋ���Ϊ���ؼ��������������Ϊ�ѵ㡢�״��㣬ע����ݲ���������c=$\frac{n}{V}$��Ӱ�����������������ѧ���ķ�����������ѧʵ��������


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 60 g | B�� | 66 g | C�� | 90 g | D�� | 184 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
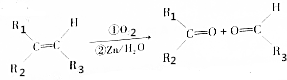
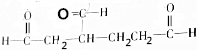 ��
�� ��1mol��
��1mol��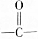 ������������������ԭ�Ӹ�����ȵ�Ԫ����C��H��
������������������ԭ�Ӹ�����ȵ�Ԫ����C��H�� �ɼ�дΪ
�ɼ�дΪ �������߱�ʾ��ѧ�����ߵĶ˵㡢�۵���ʾ̼ԭ�ӣ�̼ԭ��ʣ��Ļ��ϼ�����ԭ�Ӳ��㣮д��A���п��ܵĽṹ��ʽ��
�������߱�ʾ��ѧ�����ߵĶ˵㡢�۵���ʾ̼ԭ�ӣ�̼ԭ��ʣ��Ļ��ϼ�����ԭ�Ӳ��㣮д��A���п��ܵĽṹ��ʽ��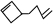 ��
��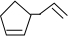 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ͷ�ϱ�[n��NO2����n��CH4��] | 400K | 500K | 600K |
| 1 | 60% | 43% | 28% |
| 2 | 45% | 33% | 20% |
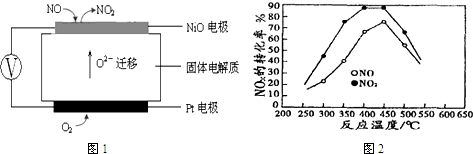
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2 CH2CH3 | B�� | CH3COOCH2CH3 | C�� | CH3CH2COOH | D�� | CH3CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
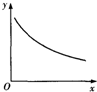 ��ij�ܱ������г���H2S��O2��������Ӧ��2H2S��g��+O2��g��?S2��g��+2H2O��g����H��0���ﵽƽ��������ı������x��ֵ�����´ﵽƽ���������y��x�仯���Ʒ���ͼ����ǣ�������
��ij�ܱ������г���H2S��O2��������Ӧ��2H2S��g��+O2��g��?S2��g��+2H2O��g����H��0���ﵽƽ��������ı������x��ֵ�����´ﵽƽ���������y��x�仯���Ʒ���ͼ����ǣ�������| ѡ�� | x | y |
| A | �¶� | �������ƽ����Է������� |
| B | ѹǿ | S2����������� |
| C | ������� | H2SŨ�� |
| D | O2Ũ�� | ƽ�ⳣ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe3+ | B�� | C032- | C�� | OH- | D�� | Mg2+ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com