
��4�֣����з����п���֤��3H2��N2  2NH3�Ѵ�ƽ��״̬����___
_____
2NH3�Ѵ�ƽ��״̬����___
_____
�ٵ�λʱ��������n mol H2��ͬʱ����n mol NH3
��һ��H��H�����ѵ�ͬʱ������H��N������
�۰ٷ���� N2%��NH3% �ܷ�Ӧ���ʦ�(H2)����3��(N2)��ʱ
��c(NH3) �� c(H2) �� c(N2)��2 �� 3�� 1ʱ
���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯
���¶Ⱥ����һ��ʱ������ѹǿ���ٱ仯
������һ������������ƽ����Է����������ٱ仯
������һ���������������������ٱ仯

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
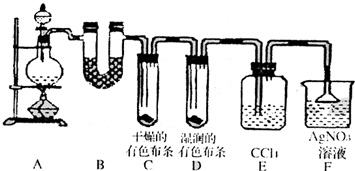 ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣮
ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣����з����п���֤��3H2��N2 2NH3�Ѵ�ƽ��״̬����___ _____
�ٵ�λʱ��������n mol H2��ͬʱ����n mol NH3
��һ��H��H�����ѵ�ͬʱ������H��N������
�۰ٷ���� N2%��NH3% �ܷ�Ӧ���ʦ�(H2)����3��(N2)��ʱ
��c(NH3)�� c(H2) �� c(N2)��2 �� 3�� 1ʱ
���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯
���¶Ⱥ����һ��ʱ������ѹǿ���ٱ仯
������һ������������ƽ����Է����������ٱ仯
������һ���������������������ٱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012������ʡ��ԭ���и߶���ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
��4�֣����з����п���֤��3H2��N2  2NH3�Ѵ�ƽ��״̬����___ _____
2NH3�Ѵ�ƽ��״̬����___ _____
�ٵ�λʱ��������n mol H2��ͬʱ����n mol NH3
��һ��H��H�����ѵ�ͬʱ������H��N������
�۰ٷ����N2%��NH3% �ܷ�Ӧ���ʦ�(H2)����3��(N2)��ʱ
��c(NH3) �� c(H2) �� c(N2)��2 ��3�� 1ʱ
���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯
���¶Ⱥ���� һ��ʱ������ѹǿ���ٱ仯
һ��ʱ������ѹǿ���ٱ仯
������һ������������ƽ����Է����������ٱ仯
������һ���������������������ٱ仯
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com