
���ڹ�ҵ�� չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����
չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д����� SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
(1)д��������ҵ���������в���SO2��ʵ����
��________________________________________________________________________��
��________________________________________________________________________��
(2)����SO2��Ⱦ�ɲ��õĴ�ʩ��(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
��___________________ _____________________________________________________��
_____________________________________________________��
(3)ʪʽʯ��ʯ—ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������ Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ—ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�________________________________________________________________________
_____________ ___________________________________________________________��
___________________________________________________________��
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����__________________��
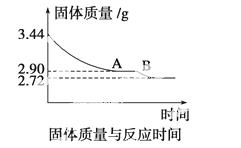
(4)ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4·xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ����ͼ��ʾ�����ݱ��������������Ϊ2.72 g���ٸı䡣��
��ʯ��Ļ�ѧʽ����ͼ����AB�ζ�Ӧ������Ļ�ѧʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧϰ��ѧ����Ҫ���ߡ�����������ʾ���ʱ仯�Ļ�ѧ��������ȷ����
A��K37ClO3��ŨHCl�ڼ�������������Cl2�Ļ�ѧ����ʽ:
K37ClO3+6HCl=K37Cl+3Cl2��+3H2O
B����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ: H2��g��+ O2��g��=H2O��g�� ��H= -241.8KJ/mol
O2��g��=H2O��g�� ��H= -241.8KJ/mol
C����1��2ml FeCl3������Һ���뵽20ml��ˮ�������������ӷ���ʽ:
Fe3++3H2O Fe(OH)3(����)+3H+
Fe(OH)3(����)+3H+
D.����Ȼ�����Һ�� 2Cl—+2H+ Cl2��+H2��
Cl2��+H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���������������ͼ���£�

��ش��������⣺
(1)�������������Ի�����Ϊԭ�ϣ������ڹ����������������Ϊԭ�ϣ�������________________________________________________________________________��
(2)������������Ӧ��ǰ�辻����ԭ����_________________________ ________
________
_____________________ ___________________________________________________��
___________________________________________________��
(3)�ڴ���Ӧ����ͨ��ʹ�ó�ѹ���ڴ�������SO2��ת����Ϊ90%�����Dz��ַ�����Ҳ�ȡ��ѹ��������ȡSO3����ȡ��ѹ��ʩ��Ŀ�ij��˼ӿ췴Ӧ�����⣬������____________________________���Ӷ��������Ч�ʡ�
(4)��ҵ�����г��ð�—�ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ�ġ��û�ѧ����ʽ��ʾ�䷴Ӧԭ����____________________________________________________
________________________________________________________________________
________________________________________________________________________��
(5)�����Ṥҵ�⣬�������ҵ������������صĹ�ҵ������������ȷ����________��
A����ˮ���壺��ˮŨ���ȿ�����ˮ������������Һ����Һ��
B����ˮ��þ����̲����ʯ��ˮMgO�ۻ����þ
C����ҵ���������NO2ˮ�������ᡪ��β������
D����ҵ�ϳɰ�����Ȼ��һ�������������ϳ�����������NH3��H2��N2ˮ����백
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ��
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��� ������ţ���
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��ء��塢þ����ȡ����
��2�����á���������������Ũ��ˮ�д���Br2�����ô������ա������������Ҫ��Ӧ��Br2+Na2CO3+H2O 
 NaBr + N
NaBr + N aBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ mol��
aBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ mol��
��3����ˮ��þ��һ�ι�����������ͼ��
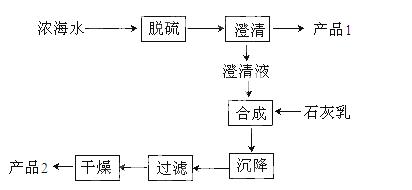
Ũ��ˮ����Ҫ�ɷ����£�
| ���� | N | Mg2+ | Cl- | SO42- |
| Ũ��/g/L | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ ����Ʒ2�Ļ�ѧʽΪ ��1LŨ��ˮ���ɵõ��� Ʒ2������Ϊ g��
Ʒ2������Ϊ g��
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ �����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������____________________________________��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����22____��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c����Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2·6H2O��
d����MgCl2·6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ����__________________��Ŀ����_________________��
��������ȡþ�������У�Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��________________________________________________________________________��
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��Ϊʲô��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4����������������Ԫ�ء��䷴Ӧԭ��Ϊ____________________________________
________________________________________________________________________(��֬��HR����)��
�������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ________________________________________________________________________
________________________________________________________________________��
(4)��˾ƥ��(COOHOOCCH3) �ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
________________________________________________________________________��
�˷�Ӧ����������________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ο��±������ʵ��۵㣬�ش��й� ���⣺
���⣺
| ���� | NaF | NaCl | NaBr | NaI | NaCl | KCl | RbCl | CsCl |
| �۵�/�� | 995 | 801 | 755 | 651 | 801 | 776 | 715 | 646 |
| ���� | SiF4 | SiCl4 | SiBr4 | SiI4 | SiCl4 | GeCl4 | SnCl4 | PbCl4 |
| �۵�/�� | -90.4 | ��70.4 | 5.2 | 120 | ��70.4 | ��49.5 | ��36.2 | ��15 |
(1)�Ƶ�±���P��������Ȼ�����۵���±�����Ӽ���������ӵ�________�йأ�����________�������۵����ν��͡�
(2)���±������۵㼰�衢�ࡢ����Ǧ���Ȼ�����۵���________�йأ�����________����________�����۵��������ߡ�
(3)�Ƶ�±������۵����Ӧ�Ĺ��±������۵�ߵö࣬����________�йأ���Ϊ_______________��
��ǰ�ߵ��۵�Զ���ں��ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
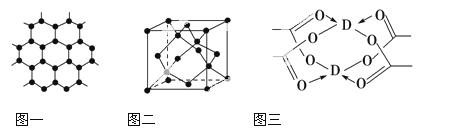
������(BN)�����ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԡ����ǵľ���ṹ����ͼ��ʾ��
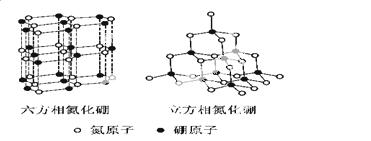
�Ż�̬��ԭ�ӵĵ����Ų�ʽΪ ��
�� ���������־����˵������ȷ���� (�����)��
a.�����൪�����ЦҼ��ͦм�������Ӳ�ȴ� b.�����൪������������С�������ʵ���
c.���־����е�B��N����Ϊ���ۼ� d.���־����Ϊ���Ӿ���
�������൪���������һ����ԭ�������ڵ�ԭ�ӹ��ɵĿռ乹��Ϊ ����ṹ��ʯī����ȴ�����磬ԭ���� ��
�������൪�������У���ԭ�ӵ��ӻ��������Ϊ ���þ������Ȼ��������ظ�ԭ����Լ300Km�Ĺŵؿ��б����֡�������һ�����γ���ʵ���ƶ�ʵ�����������൪����ϳ������൪������Ҫ������Ӧ�� ��
��NH4BF4(�������)�Ǻϳɵ��������ܵ�ԭ��֮һ��1mo NH4BF4���� mol��λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ����Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������η��ӣ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ�
��ش��������⣺
(1)������Ԫ���е縺������Ԫ�أ����̬ԭ�ӵļ۵����Ų�ͼΪ________����һ��������С��Ԫ����________(��Ԫ�ط���)��
(2)C���������ǰ����Ԫ�طֱ���A�γɵĻ�����е��ɸߵ��͵�˳����________(�ѧʽ)��������˵ݱ���ɵ�ԭ����___________________��
(3)BԪ�ؿ��γɶ��ֵ��ʣ�һ�־���ṹ��ͼһ��ʾ����ԭ�ӵ��ӻ�����Ϊ________����һ�ֵľ�����ͼ����ʾ�����˾����е��ⳤΪ356.6 pm����˾������ܶ�Ϊ________________________________________________g·c m��3(������λ��Ч����)��(
m��3(������λ��Ч����)��(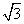 ��1.732)
��1.732)
(4)DԪ���γɵĵ��ʣ��侧��Ķѻ�ģ��Ϊ________��D�Ĵ����ξ���ֲ��ṹ��ͼ�����þ����к��еĻ�ѧ����________(��ѡ�����)��
�ټ��Լ������ڷǼ��Լ���������λ�������ܽ�����
(5)��D����������Һ�еμӹ�����ˮ���۲쵽��������________����д���������̵����ӷ���ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1.28 gij���庬�еķ�����ĿΪ1.204��1 022����������Ħ������Ϊ(����)
022����������Ħ������Ϊ(����)
A��64 g B. 64
C��64 g/mol D��32 g/mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com