
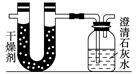
| | ʵ��ǰ | ʵ��� |
| (�������U�ι�)������ | 101.1 g | 102.9 g |
| (ʯ��ˮ�����ƿ)������ | 312.0 g | 314.2 g |
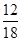 ��1.8 g��
��1.8 g��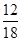 ��0.2 g
��0.2 g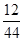 ��2.2 g��
��2.2 g��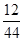 ��0.6 g
��0.6 g ��
�� ��1��4��
��1��4��

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���� | �Լ� | װ�� |
| A | C2H6(C2H4) | �� | �� |
| B | ��(����) | �� | �� |
| C | CH3COOC2H5(CH3COOH) | �� | �� |
| D | �ױ�(���ױ�) | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H5OH | B��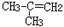 | C��CH3CH2CH2COOH | D��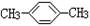 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H6 | B��C3H8 | C��CH3CH2CH2CH3 | D��CH3CH2OH |
�鿴�𰸺ͽ���>>
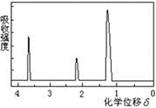
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
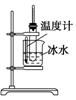 | 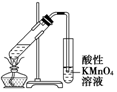 |  | 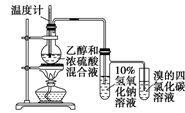 |
| A��ʵ������ȡ������������ | B��֤�������顢NaOH���Ҵ���Һ����������ϩ | C����������������̼���ƺ�ˮ�Ļ���� | D��֤���Ҵ���Ũ���Ṳ��������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C3H8��C4H6 | B��CH4O��C3H4O5 |
| C��C2H6��C4H6O2 | D��C3H6��C4H6O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ͨʽΪCnH2n+2,��nֵ����,̼Ԫ�ص������ٷֺ�����С |
| B����ϩ����ˮ�����ӳɷ�Ӧ�IJ���Ϊ������ |
| C��1 mol��ǡ����3 mol������ȫ�ӳ�,˵��һ����������������̼̼˫�� |
| D��ͨʽΪCnH2n+2��n=7,��������5��̼ԭ�ӵ���������5�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com