
������������PBT��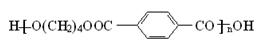 ������Ҫԭ��G��1��4����������������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ�����֪B������ԭ�Ӵ���ͬһƽ�档
������Ҫԭ��G��1��4����������������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ�����֪B������ԭ�Ӵ���ͬһƽ�档

���������������й���Ϣ���ش����⡣
��CH2=CH-CH=CH2��Br2��CCl4��Һ����1��1�ӳɿɵõ�BrCH2-CH=CH-CH2Br��BrCH2-CHBr-CH=CH2���ֲ��
��2R��Cl��2Na R��R��2NaCl
![]()
��
����
��1��д��E��F�Ľṹ��ʽ�� �� ��
��2��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ�� ��
��3��������Ƴ���B��C����D�ķ�Ӧ����ͼ���л����ýṹ��ʽ��ʾ������ע����Ӧ��������
��ʾ���ٺϳɹ��������Լ���ѡ �ڷ�Ӧ����ͼ��ʾ����ʾ�����£�
B C ���� G
��1��CH2=CHCl HOCH2C��CCH2OH ��3�֣�
��2��![]() ��2�֣�
��2�֣�
��3��CH��CCH=CH2 ![]() CH2=CHCH=CH2
CH2=CHCH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br![]() Br��CH2��4Br
Br��CH2��4Br![]() HO��CH2��4OH ��3�֣�
HO��CH2��4OH ��3�֣�
������һ��������л��ƶ��⣬��Ŀ��Ϣ������������Ϣ���ɿ�ͼ��Ϣ���ƶϷ�ʽ���������Ƶ������������Ƶ���ͻ�ƿ�Ҳ�����Σ����һ���л��ϳɹ��̺ϳ�·�ߵ�ѡ�����ƣ����м�ǿ�Ŀ����Ժ��ۺ��ԣ��Ƚ���ӱ�������˿���˵����Ӧ����ѧ������Ҫ����һ���ۺϿ����л���ѧ����֪ʶ��˼ά�����ĺ��⡣������Ŀ��������Ϣ֪��AΪHC��CH��BΪHC��CCH��CH2��CΪCH2=CHCH=CH2��DΪBr��CH2��4Br��EΪCH2=CHCl��FΪHOCH2C��CCH2OH��GΪHO��CH2��4OH��������Ŀ�����ķ�Ӧ��Ϣ���л���ѧ����֪ʶ��������Ƴ���B��C����D�ķ�Ӧ����ͼ��CH��CCH=CH2 ![]() CH2=CHCH=CH2
CH2=CHCH=CH2![]() CH2BrCH=CHCH2Br
CH2BrCH=CHCH2Br![]() Br��CH2��4Br
Br��CH2��4Br![]() HO��CH2��4OH
HO��CH2��4OH


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��Ӣ����ѧ2007�������ѧ�ڶ���ϰר��ǿ��ѵ��10 ���ͣ�022
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]() (R������������ͬ)
(R������������ͬ)
R��C��C����CH2��n��CHCH2+H2![]() R��CHCH����CH2��n��CHCH2
R��CHCH����CH2��n��CHCH2
1��4-��������������������PBT���۶Ա������ᶡ����������Ҫԭ�ϣ�������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ�����д����Ӧ���ʵĽṹ��ʽ��

�����
��1��A��E�Ľṹ��ʽ��_______________��_______________��
��2��д������CH2BrCH=CHCH2Br�Ļ�ѧ��Ӧ����ʽ��______________________________
д������F��PBT���Ļ�ѧ��Ӧ����ʽ��_________________________________________��
��3��ijѧ���о���������Ȳ���Ƶ��Ҷ�����������Ƴ������ķ�Ӧ����ͼ��
��ʾ���ٺϳɹ��������Լ���ѡ
�ڷ�Ӧ����ͼ��ʾ����ʾ�����£�
a![]() b
b![]() c����
c����![]() �Ҵ�
�Ҵ�
______________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
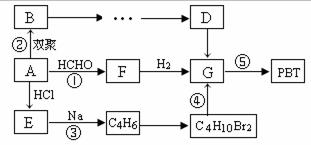
������������PBT ��![]() �� ����Ҫԭ��G��1��4-��������������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ�����֪AΪ��Ȳ��B������ԭ�Ӿ�����ͬһƽ���ϡ�
�� ����Ҫԭ��G��1��4-��������������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ�����֪AΪ��Ȳ��B������ԭ�Ӿ�����ͬһƽ���ϡ�
��������������Ϣ���ش��й����⡣
I�� CH2=CH-CH=CH2��Br2��CCl4��Һ����1��1�ӳɷ�Ӧ���ɵõ�BrCH2-CH=CH-CH2Br��BrCH2-CHBr-CH=CH2���ֲ��
II��2R��Cl + 2Na ![]() R��R + 2NaCl
R��R + 2NaCl
III��R1��C��C��H+R2��CHO ![]()
![]() ��R������ͬ������H��
��R������ͬ������H��
IV��R��C��C����CH2��n��CH=CH2+H2 ![]() R��CH=CH-��CH2��n��CH=CH2��n��0��
R��CH=CH-��CH2��n��CH=CH2��n��0��
��1���ṹ��ʽ��EΪ_________________��FΪ___________________��
��2����Ӧ�ݵĻ�ѧ��Ӧ����ʽΪ��
____________________________________________________________________��
��3������Ƴ���B������G�ķ�Ӧ����ͼ���л����ýṹ��ʽ��ʾ����ע����Ӧ��������
��ʾ���ٺϳɹ��������Լ���ѡ �ڷ�Ӧ����ͼ��ʾ����ʾ�����£�
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08ɽ��ʵ����ѧ��ģ�����л���ѧ������8�֣�
������������PBT(H+O(CH2)4OOC�D�DCO+nOH)����Ҫԭ��G��1��4��������������ͨ����ͼ���ֲ�ͬ�ĺϳ�·���Ʊ���֪AΪ��Ȳ��B������ԭ�Ӿ�����ͬһƽ����

��������������Ϣ���ش��й�����
��CH2=CH-CH=CH2��Br2��CCl4��Һ����1:1�ӳɷ�Ӧ���ɵõ�BrCH2-CH=CH-CH2Br��BrCH2-CHBr-CH=CH2���ֲ���

![]()
��1���ṹ��ʽ��EΪ_______________��FΪ_______________��
��2����Ӧ�ݵĻ�ѧ��Ӧ����ʽΪ��
____________________________________________________��
��3������Ƴ��ԡ�������G�ķ�Ӧ����ͼ���л����ýṹ��ʽ��ʾ����ע����Ӧ������
��ʾ���ٺϳɹ��������Լ���ѡ �ڷ�Ӧ����ͼ��ʾ����ʾ�����£�
![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com