
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1 817 | 2 745 | 11 578 |
| B | 738 | 1 451 | 7 733 | 10 540 |
| ���ۼ� | C��C | C��N | C��S |
| ����/kJ��mol��1 | 347 | 305 | 259 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��NaN3��KN3�ṹ���ƣ�ǰ�߾����ܽ�С |
B������صľ����ṹ��ͼ��ʾ�� ��ÿ�������з�̯2����ԭ�� ��ÿ�������з�̯2����ԭ�� |
| C�����ĵ�һ�����ܴ����� |
| D�����������º��ȶ�������Ϊ���ĵ縺��С |
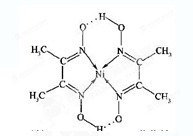
 Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
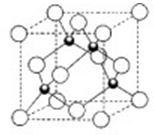
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | A | B | D | E |
| ���ϼ� | -4 | -2 | -3 | -2 |
| �縺�� | 2.5 | 2.5 | 3.0 | 3.5 |
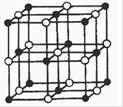
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | H2 | O2 | F2 | OH | OF | HF |
| E/(kJ/mol) | 432 | 494 | 155 | 424 | 220 | 566 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ | B��CH3OH���״��� | C��CS2�� | D��CH3COOH�����ᣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com