��2009?������һģ��M��R���������г����Ľ������ʣ�����R���������Ľ������ס����ǻ�������м��Ǻ�ɫ���壬����R��X��ȼ�յõ���
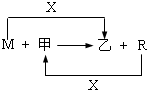
��1��M����ڸ����·�Ӧ�Ļ�ѧ����ʽ��
��
��2����ⷨ��R�ͼ����������װ����ͼ��a��4mol?L
-1NaCl��1mol?L
-1NaOH�Ļ����Һ��
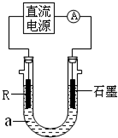
������aʱ��Ҫ��ȥ����ˮ���ܽ��O
2��������
���
���
�ķ�����
��ʯī�缫Ӧ���Դ��
��
��
��������������������ӣ�ͨ���R�缫������������
���ɰ�ɫ����
���ɰ�ɫ����
��R���ĵ缫��Ӧʽ��
Fe-2e-+2OH-=Fe��OH��2��
Fe-2e-+2OH-=Fe��OH��2��
��
��ֹͣʵ��һ��ʱ�����R���ϲ��к��ɫ���ʲ�������Ӧ�Ļ�ѧ����ʽ��
4Fe��OH��2 +O2+2H2O�T4Fe��OH��3
4Fe��OH��2 +O2+2H2O�T4Fe��OH��3
��
��3����R��ij�������ĩ��M��ĩ��Ϻ�ֳ����ȷݣ�һ���ڸ�����ǡ����ȫ��Ӧ�������������ᷴӦ����һ��ֱ�ӷ����������ռ���Һ�г�ַ�Ӧ��ǰ��������������ɵ�������������a��b����R��������Ļ�ѧʽ��
FeaOb
FeaOb
��





 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�