
��һ���¶��£���һ2 L�̶��ݻ����ܱ�������ͨ��20 mol��N2��60 mol��H2������5���Ӻ�N2��Ũ����5 mol/L���ֹ���5����N2��H2��NH3��Ũ�Ȳ��ٱ仯����ʱNH3��Ũ����14 mol/L���ﵽƽ��ų�QKJ��������������������⣺
(1)ǰ5������H2��ʾ�Ļ�ѧ��Ӧ����(����)��
(2)��Ӧ�ﵽƽ���N2��ת����(����)��
(3)��ʾ���¶��ºϳɰ��Ļ�ѧƽ�ⳣ���ı���ʽΪ(����)��
(4)��ƽ���������ѹǿ����ѧƽ����(����)�����ƶ�(��������桱������)��
(5)���¶��·�Ӧ���Ȼ�ѧ����ʽΪ(����)(�ú�Q��ʽ�ӱ�ʾ)��
(6)�ڸ��¶��£�����һ2 L�̶��ݻ����ܱ�������ͨ��N2��5 mol��H2��15 mol��NH3��30 mol����Ӧ�ﵽƽ���H2��Ũ����(����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���һ�ܱ���ϵ�з������з�Ӧ��N2(g)+3H2(g) 2NH3(g)����H<0 ��
�ش��������⣺
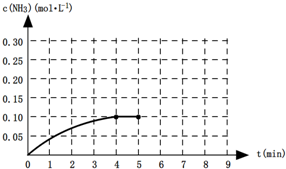
(1)��һ���¶��£���һ�ݻ�Ϊ2L���ܱ�������ͨ��0.3molN2��0.9molH2,2min�ﵽƽ��ʱ��C(N2)Ϊ0.1mol/L����H2��ת����Ϊ____________________����H2Ũ�ȱ仯��ʾ��ƽ��
��Ӧ����Ϊ__ ____ �����¶��µ�ƽ�ⳣ��K= ____________��
(2)��Ҫʹ��Ӧ��ʼʱ���淴Ӧ������У��Ҵﵽƽ������������ʵ�����ƽ��״̬(1)��ͬ������ʼʱn(NH3)��ȡֵ��ΧΪ ___________________ ��
(3)��ͼ��ijһʱ�������-ʱ������ͼ��
��ͼ��t1ʱ�̽����������Ϊԭ����1/2������t2ʱ���ٴδﵽƽ��ʱN2��Ũ��Ϊԭƽ���1.9������ƽ�� _____
�������ơ������ơ������ƶ�������
������ͼ�л���t1��t2ʱ��η�Ӧ���ʱ仯�����
��t3��t5ʱ����ϵ�����ı��ijһ������ ���ǣ�__________________________��_______________�����б�ʾƽ��������NH3�ĺ�����ߵ�ʱ�����___________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ϲ����и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣���һ�ܱ���ϵ�з������з�Ӧ��N2(g)+3H2(g) 2NH3(g)����H<0 ��
2NH3(g)����H<0 ��
�ش��������⣺
(1)��һ���¶��£���һ�ݻ�Ϊ2L���ܱ�������ͨ��0.3molN2��0.9molH2,2min�ﵽƽ��ʱ��C(N2)Ϊ0.1mol/L����H2��ת����Ϊ____________________����H2Ũ�ȱ仯��ʾ��ƽ��
��Ӧ����Ϊ__ ____ �����¶��µ�ƽ�ⳣ��K= ____________��
(2)��Ҫʹ��Ӧ��ʼʱ���淴Ӧ������У��Ҵﵽƽ������������ʵ�����ƽ��״̬(1)��ͬ������ʼʱn(NH3)��ȡֵ��ΧΪ ___________________ ��
(3)��ͼ��ijһʱ�������-ʱ������ͼ��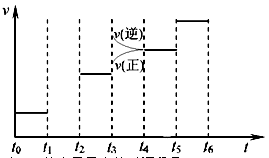
��ͼ��t1ʱ�̽����������Ϊԭ����1/2������t2ʱ���ٴδﵽƽ��ʱN2��Ũ��Ϊԭƽ���1.9������ƽ�� _____
�������ơ������ơ������ƶ�������
������ͼ�л���t1��t2ʱ��η�Ӧ���ʱ仯�����
��t3��t5ʱ����ϵ�����ı��ijһ������ ���ǣ�__________________________��_______________�����б�ʾƽ��������NH3�ĺ�����ߵ�ʱ�����___________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣���һ�ܱ���ϵ�з������з�Ӧ��N2(g)+3H2(g)  2NH3(g)����H<0 ��
2NH3(g)����H<0 ��
�ش��������⣺
(1)��һ���¶��£���һ�ݻ�Ϊ2L���ܱ�������ͨ��0.3molN2��0.9molH2,2min�ﵽƽ��ʱ��C(N2)Ϊ0.1mol/L����H2��ת����Ϊ____________________����H2Ũ�ȱ仯��ʾ��ƽ��
��Ӧ����Ϊ__ ____ �����¶��µ�ƽ�ⳣ��K= ____________��
(2)��Ҫʹ��Ӧ��ʼʱ���淴Ӧ������У��Ҵﵽƽ������������ʵ�����ƽ��״̬(1)��ͬ������ʼʱn(NH3)��ȡֵ��ΧΪ ___________________ ��
(3)��ͼ��ijһʱ�������-ʱ������ͼ��
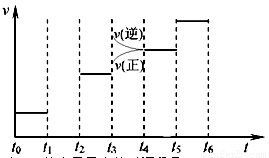
��ͼ��t1ʱ�̽����������Ϊԭ����1/2������t2ʱ���ٴδﵽƽ��ʱN2��Ũ��Ϊԭƽ���1.9������ƽ�� _____
�������ơ������ơ������ƶ�������
������ͼ�л���t1��t2ʱ��η�Ӧ���ʱ仯�����
��t3��t5ʱ����ϵ�����ı��ijһ������ ���ǣ�__________________________��_______________�����б�ʾƽ��������NH3�ĺ�����ߵ�ʱ�����___________.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com