
(1)��ͼ��������A��B�ͽ������װ���ռ�NO����(������A�Ѿ����������Լ�飻�ڳ�ˮ�ⲻ��ѡ�������Լ�)����ȷ�IJ��������ǣ�________��

(2)��һ����NO���Թܵ�����ˮ���У�Ȼ�����Թ���ͨ��һ������O2���Թ���ǡ�ó���ˮʱ����ͨ���O2��ԭNO����������Ϊ________�����������ɵ����ʲ�������ɢ����Ϊ��״���������õ���Һ�����ʵ���Ũ��ӦΪ________��
(3)�����£���ʢ��10 mL��NO2��10 mL��NO�������Ĵ��Թܵ�����ˮ�У��������л���ͨ��O2һ��ʱ����Թ��ڲ���2 mL���壬��ͨ��O2���������Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ?mol-1���ش��й��кͷ�Ӧ�����⣮
��֪H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ?mol-1���ش��й��кͷ�Ӧ�����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
��֪H��(aq)��OH��(aq)=H2O(l) ��H����57.3 kJ��mol��1���ش��й��кͷ�Ӧ�����⡣
��1����0.1 mol Ba(OH)2���ϡ��Һ������ϡ���ᷴӦ���ܷų�________kJ������
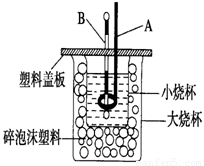
��2����ͼװ��������A�������� ������ĭ���ϵ������� ��
��3����ͨ��ʵ��ⶨ�к��ȵĦ�H�������������ڣ�57.3 kJ��mol��1����ԭ�������
��
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� (�ƫ����ƫС��������Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ���ݰ��أ��У�һ�и߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com