
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
��2������������Һ���ѡ����ʵ�����ƿ��������Һϡ�������������ʵ����ʵ������������ҪŨ��������������Ũ�������ѡ����ʵ���Ͳ��
��3����������ƿ������
��4���������������ʵ����ʵ���n����Һ���V��Ӱ�죬����C=$\frac{n}{V}$���з���������ʹnƫ����ʹVƫС�IJ���������ʹ��ҺŨ��ƫ�ߣ���֮����ҺŨ��ƫ�ͣ�
��� �⣺��1���ܶ�Ϊ1.84g•cm-3�����ʵ���������Ϊ98%���������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L��
�ʴ�Ϊ��18.4mol/L��
��2������250mL���ʵ���Ũ��Ϊ0.46mol•L-1�����ᣬӦѡ��250mL����ƿ��
����ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ�����������V��18.4mol/L=0.46mol/L
��250mL�����V=6.25mL������Ӧѡ��10mL��Ͳ��
��ѡ���٢ݣ�
��3������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ�
�ʴ�Ϊ���¶ȡ��������̶��ߣ�
��4��A��������ƿ��ת����Һʱ������Һ�彦�������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A��ѡ��
B��δϴ��ϡ��ŨH2SO4��С�ձ������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���B��ѡ��
C������ʱ���ӿ̶��ߣ�������Һ�����ƫС����ҺŨ��ƫ�ߣ���Cѡ��
D��ϴ������ƿδ���T����������Һ�������ʵ����ʵ�������Һ�������������Ӱ�죬��ҺŨ�Ȳ��䣬��D��ѡ��
E�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ͣ���E��ѡ��
��ѡ��C��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������Ʋ��������ǽ���ؼ���ע������ƿ����Ͳ�Ĺ��ѡ��ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ƕ�����ˮ��Ӧ���ɶ�Ӧ���� | |
| B�� | ��״�������Ƕ�����ɫ��ζ������ | |
| C�� | ��������Ԫ�ص�����������NO��NO2��SO2 | |
| D�� | ʵ���ҿ�������������ԭ��Ӧ�ֱ��Ʊ�NO��NO2��SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��һ���¶��£���1L����̶����ܱ������м���1molA��g����������Ӧ2A��g��?B��g��+C��g����B�����ʵ�����ʱ��ı仯��ͼ��ʾ�� 0-2min�ڵ�ƽ����Ӧ����v��A��=0.1mol/��L•min������ͬ�¶��£�����ʼ����A��g�������ʵ�����ԭ����2������ƽ��ʱbe��ԭ����2����
��һ���¶��£���1L����̶����ܱ������м���1molA��g����������Ӧ2A��g��?B��g��+C��g����B�����ʵ�����ʱ��ı仯��ͼ��ʾ�� 0-2min�ڵ�ƽ����Ӧ����v��A��=0.1mol/��L•min������ͬ�¶��£�����ʼ����A��g�������ʵ�����ԭ����2������ƽ��ʱbe��ԭ����2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
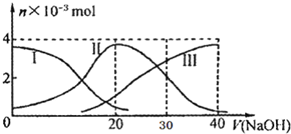 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�жϣ�����˵������ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�жϣ�����˵������ȷ���ǣ�������| A�� | H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A�TH++HA-��HA-�TH++A2- | |
| B�� | ��V��NaOH��=20mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� | |
| C�� | �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮС | |
| D�� | ��V��NaOH��=30mLʱ����Һ�д������¹�ϵ��2c��H+��+c��HA-��+2c��H2A��=c��A2-��+2 c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����
��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뾶��С��S2-��Cl-��Na+��Al3+��F- | |
| B�� | ���ȶ��Դ�С��SiH4��PH3��NH3��H2O��HF | |
| C�� | �ܶȴ�С��Rb��K��Na��Li | |
| D�� | �����ʵ���Ũ����Һ������ǿ��˳��HClO4��H2SO4��H3PO4��H2SiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
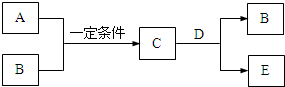 A��B��C��D��E����ѧ��ѧ�еij������ʣ�A��B�Ƕ�����Ԫ����ɵĵ��ʣ���ת����ϵ��ͼ��
A��B��C��D��E����ѧ��ѧ�еij������ʣ�A��B�Ƕ�����Ԫ����ɵĵ��ʣ���ת����ϵ��ͼ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com