
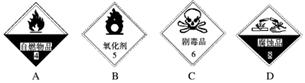
��������ѧ��ѧʵ���ҵij���ҩƷ���������Т����ԡ�����ˮ�ԡ�����ˮ�ԡ���ǿ�����ԡ��ݴ����ã��뽫���������Ӧ�ĺ����ϣ�
(1)п��ϡH2SO4��H2________��
(2)Ũ�����������________��
(3)Ũ���������ǵ�̿��ʵ��(�����ʵ��)________��
(4)ʵ�������Ҵ��ͱ�������ȡ��������________��
(5)����������ˮ��________��
(6)��ά�ص�ˮ��________��
(7)Ũ������ͭ�ķ�Ӧ________��
(8)Ũ����ʹʪ��ʯ����ֽ��죬�����ֱ��________��
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ɵ͵��ߵ�˳��������ȷ��һ����(����)
A��1s��2p��3d��4s B��1s��2s��3s��2p
C��2s��2p��3s��3p D��4p��3d��4s��3p
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʰ��۵��ɸߵ������е�˳����ȷ����(����)
A��NaCl��SiO2��CO2��Na B��Na��NaCl��CO2��SiO2
C��CO2��Na��NaCl��SiO2 D��SiO2��NaCl��Na��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2����������ȷ����(����)
A��SO2ͨ����ˮ����Һ��ɫ�����Լ���
B�����������������ˮ���ȶ���������
C��SO2����ͨ��NaOH��Һһ���õ�Na2SO3
D��S��SO2��SiO2�������ʾ�����NaOH��Һ��Ӧ������������ijЩ�ᷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)���������У����γ��������______��
A���������� B�����ȴ���
C��������̼ D������
(2)�������¼��ִ�ʩ���ٶ�ȼ��úʱ������β�����г�������������ԭú��ȼ�ϣ���ȼúʱ���������������ܿ��������Դ�������ܼ�����������Ĵ�ʩ��______��
A���٢ڢ� B���ڢۢ�
C���٢ڢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�
����һ
(1)Ũ�����Լ�ƿ���ʺ����ϵı�ǩ��________(�����)��
(2)��ͬѧȡ6.4 gͭƬ��10 mL 18 mol·L��1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ������Ұ�ɫ��������ͬѧΪ����֤���лҰ�ɫ��������Ҫ�ɷ֣��������ʵ�飺
ʵ�鲽�裺�㵹���ϲ�Һ��������ûҰ�ɫ�Ĺ����м�����������ˮ���ӱ߽��衣
ʵ������________________________________________________________��
ʵ����ۣ����ûҰ�ɫ����Ļ�ѧʽΪ__________��
(3)�һ��۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ������ɫ������ʧ��ԭ����(�û�ѧ����ʽ�ش�)_____________________________ _____________________________________��
ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�࣬�Ҹ����Լ���ѧ�Ļ�ѧ֪ʶ����Ϊ�Թ��л�������ʣ�ࡣ��������Ϊ��������______________________________________��
������
(4)��ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·��2Cu��O2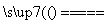 2CuO��CuO��H2SO4===CuSO4��H2O��
2CuO��CuO��H2SO4===CuSO4��H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ���
��________________________________________________________________________��
��________________________________________________________________________��
������
(5)��ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ������Һ����ɫ��д����Ӧ�Ļ�ѧ����ʽ_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
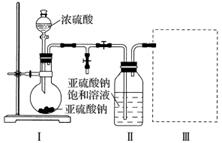
(1)װ�â��в�������Ļ�ѧ����ʽΪ__________________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����________________________________________________________________________
________________________________________________________________________��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ__________(�����)��
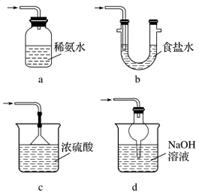
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����_____(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����_____(�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������R2����ԭ�Ӻ�����n�����ӣ�Rԭ�ӵ�������Ϊm����� g Rԭ����ȫת��ΪR2��ʱ�����е��ӵ����ʵ����� (����)
A.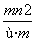 mol B.
mol B.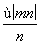 mol
mol
C����(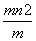 ) mol D.
) mol D.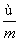 (m��n��2) mol
(m��n��2) mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(��NOx��ʾ)�Ǵ�����Ⱦ����Ҫ���أ�����NOx�������ص㣬���������ֻ�ѧ����������������Ⱦ�ķ�����
(1)�ð��ɽ���������ת��Ϊ�����塣��֪��4NH3��6NO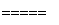 5N2��6H2O,8NH3��6NO2
5N2��6H2O,8NH3��6NO2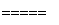 7N2��12H2O��ͬ��ͬѹ�£�3.5 L NH3ǡ�ý�3.0 L NO��NO2�Ļ��������ȫת��ΪN2����ԭ���������NO��NO2�����֮����________��
7N2��12H2O��ͬ��ͬѹ�£�3.5 L NH3ǡ�ý�3.0 L NO��NO2�Ļ��������ȫת��ΪN2����ԭ���������NO��NO2�����֮����________��
(2)��ҵβ���е��������ﳣ���ü�Һ���շ�������
��NO2���ռ���Һ����ʱ�������������Σ������ʵ���֮��1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________��
��NO��NO2�����ʵ���֮��1��1������NaOH��Һ��ȫ���պ�ֻ�õ�һ�����Σ������εĻ�ѧʽ��______��
(3)������ҺҲ�����ڴ��������������������Ӧԭ��������(2)���ƣ�ͬʱ����CO2��
����д��������Һ����NO2�Ļ�ѧ����ʽ��________��
������һ������ij��ҵ���������к���3.36 L NO2��1.12 L NO(�ѻ���Ϊ��״�������費��N2O4)��ǡ�ñ�200 mL̼������Һ���գ������ε����ʵ����ֱ�Ϊ________��________����̼������Һ�����ʵ���Ũ��Ϊ____________mol·L��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com