
��֪�ס������������������ܶȵĹ�ϵ���±���
������������ | ����Һ�ܶȣ�g/cm3�� | ����Һ�ܶ�(g/cm3) |
1% | 0.95 | 1.02 |
5% | 0.92 | 1.04 |
10% | 0.90 | 1.07 |
�����ʵ�1%����Һ��9%����Һ�������ϣ������ʵ�1%����Һ��9%����Һ�������Ϻ�����������ȷ����
A.��Ϻ�ס�����Һ��������������5%
B.��Ϻ�����Һ������������5%������Һ��������С��5%
C.��Ϻ����Һ������������5%������Һ��������С��5%
D.��Ϻ�ס�����Һ��������������5%
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(20��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�(1) ��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2) ��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��_____________��
(3) ��¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g)Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
�� �¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ__________�����������С�����䡱����
�� 1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1 mol��L-1��������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬____________����ǡ��������ж�������______
____________________________________________________________��
(4) ����ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
�� ��װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ
_____________________________________��
�� ��װ����a���ϵĵ缫��ӦʽΪ__________________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
�� ������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ__________________________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�
(1)��25�桢101kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2)��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8 kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��________kJ��mol��1��
��3������ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
�ټ�װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ_____________��
����װ����a���ϵĵ缫��ӦʽΪ____________________________��
����a������112mL(��״��)���壬���װ��������CH4________mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
��������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�����и���12���¿���ѧ�Ծ� ���ͣ������
(15��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�
(1)��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2)��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8 kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g)���ʱ䦤H��________kJ��mol��1��
��3������ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
�ټ�װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ_____________��
����װ����a���ϵĵ缫��ӦʽΪ____________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
��������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڼ���ѧϰЧ����⿼�Ի�ѧ�Ծ� ���ͣ������
(20��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�(1) ��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2) ��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8 kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��_____________��
(3) ��¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g) Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
�� �¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ__________�����������С�����䡱����
�� 1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1 mol��L-1��������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬____________����ǡ��������ж�������______
____________________________________________________________��
(4) ����ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��

�� ��װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ
_____________________________________��
�� ��װ����a���ϵĵ缫��ӦʽΪ__________________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
�� ������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ__________________________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и���12���¿���ѧ�Ծ� ���ͣ������
(15��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�
(1)��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2)��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8 kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��________kJ��mol��1��
��3������ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
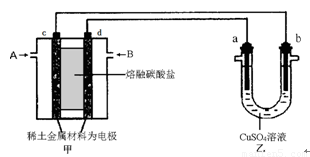
�ټ�װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ_____________��
����װ����a���ϵĵ缫��ӦʽΪ____________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
��������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com