
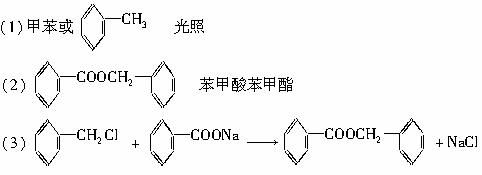
 ������9�ֻ������ת���ϵ��
������9�ֻ������ת���ϵ�� 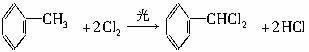 ?
?
��1�����������������������������������Ӧ������A������������?
��2��������ݸ��߿�����Ĵ���ʧȥˮ���ɻ�����ᣬ���Ľṹ��ʽ�� �������� ����3�������������Ҫ�Ķ���������Ϲ�ҵ�ϳ��û�����ں͢�ֱ�Ӻϳ�����
�˷�Ӧ�Ļ�ѧ����ʽ����������������������������������
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

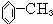
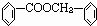
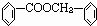
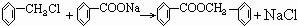
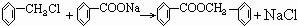
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| Ũ���� |
| �� |
| Ũ���� |
| �� |
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
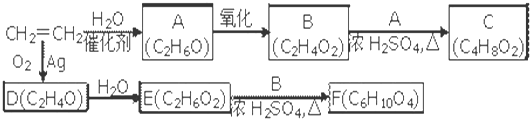
ͨ������£�����ǻ�����ͬһ��̼ԭ���ϵķ��ӽṹ�Dz��ȶ��ģ������Զ�ʧˮ������̼��˫���Ľṹ��![]()
������9���������ת���ϵ��
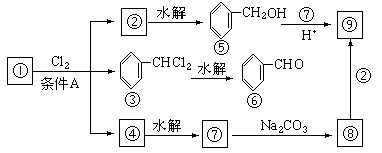
��1�����������__________����������������Ӧ������A��__________��
��2��������ݸ��߿�����Ĵ���ȥˮ���ɻ�����ᣬ ��Ľṹ��ʽ�ǣ�_______��������______________________________________��
��3�������������Ҫ�Ķ���������Ϲ�ҵ�ϳ��û������͢�ֱ�Ӻϳ�����д���˷�Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꼪��ʡ���������ѧУ�߶���ѧ����ĩ���Ի�ѧ�Ծ��� ���ͣ������
��ͨ������£�����ǻ�����ͬһ��̼ԭ���ϵķ��ӽṹ�Dz��ȶ��ģ������Զ�ʧˮ����̼��˫���ṹ��
������9�����ʵ�ת����ϵ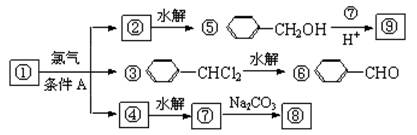
��1����������� ����������������Ӧ�������� ��
��2���ߵĽṹ��ʽ ��������ݸ��߿�����Ĵ���ȥˮ���ɻ�����ᣬ��Ľṹ��ʽ��
��3�������������Ҫ�Ķ���������Ϲ�ҵ�ϳ��âں͢�ֱ�Ӻϳ������˷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���ݵ�ͬ���칹��ܶ��֣�д���������ڲ�ͬ������廯���������ͬ���칹��Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com