
ͼ��A��Ϊ��25mL 0.1 mol��L-1 NaOH��Һ����εμ�0.2 mol��L-1 CH3COOH��Һ�����У���pH�Ƹ��ٲ����ҺpH�ı仯���ߡ���ش�
��1�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�� ����ѡ����ĸ��
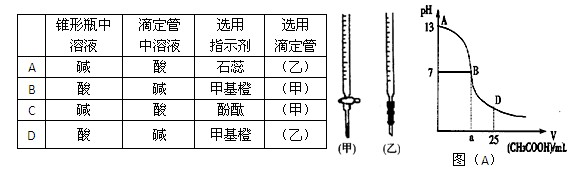
ͼ��A����B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ�� ��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ� ���䣨����ȷ�����ʲ��𣩡�
AB�����ڣ�����A��B���㣩����Һ����������Ũ�ȵĴ�С��ϵ������_____��______��
��D��ʱ����Һ��c��CH3COO����+c��CH3COOH�� 2c��Na+�������������������������
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ��ƿ����Һ | �ζ��� ����Һ |
ѡ�� ָʾ�� |
ѡ�� �ζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ��̪ | ���ң� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11�֣�ͼ��A��Ϊ��25mL 0.1 mol��L-1 NaOH��Һ����εμ�0.2 mol��L-1 CH3COOH��Һ�����У���pH�Ƹ��ٲ����ҺpH�ı仯���ߡ���ش�
��1�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�� ����ѡ����ĸ��
��2��ͼ��A����B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ�� ��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ� ���䣨����ȷ�����ʲ��𣩡�
��3��AB�����ڣ�����A��B���㣩����Һ����������Ũ�ȵĴ�С��ϵ������_____��______��
��4����D��ʱ����Һ��c��CH3COO����+c��CH3COOH�� 2c��Na+�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ��㶫ʡ�������и߶���ѧ����ĩ���Ի�ѧ�Ծ��� ���ͣ�ʵ����
��11�֣�ͼ��A��Ϊ��25mL 0.1 mol��L-1 NaOH��Һ����εμ�0.2 mol��L-1 CH3COOH��Һ�����У���pH�Ƹ��ٲ����ҺpH�ı仯���ߡ���ش�
��1�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�� ����ѡ����ĸ��
��2��ͼ��A����B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ�� ��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ� ���䣨����ȷ�����ʲ��𣩡�
��3��AB�����ڣ�����A��B���㣩����Һ����������Ũ�ȵĴ�С��ϵ������_____��______��
��4����D��ʱ����Һ��c��CH3COO����+c��CH3COOH�� 2c��Na+�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��� ���ͣ�ʵ����
��11�֣�ͼ��A��Ϊ��25mL 0.1 mol��L-1 NaOH��Һ����εμ�0.2 mol��L-1 CH3COOH��Һ�����У���pH�Ƹ��ٲ����ҺpH�ı仯���ߡ���ش�
��1�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�� ����ѡ����ĸ��

��2��ͼ��A����B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ�� ��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ� ���䣨����ȷ�����ʲ��𣩡�
��3��AB�����ڣ�����A��B���㣩����Һ����������Ũ�ȵĴ�С��ϵ������_____��______��
��4����D��ʱ����Һ��c��CH3COO����+c��CH3COOH�� 2c��Na+�������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com