
 3Fe+4CO2���У�______����������______�ǻ�ԭ����______Ԫ�ر�������
3Fe+4CO2���У�______����������______�ǻ�ԭ����______Ԫ�ر������� 3Fe+4CO2���÷�Ӧ�еĻ��ϼ۱仯ΪFe3O4 ��Fe����Ԫ����+
3Fe+4CO2���÷�Ӧ�еĻ��ϼ۱仯ΪFe3O4 ��Fe����Ԫ����+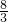 �ۡ�0�ۣ��õ��ӻ��ϼ۽��ͣ�����Fe3O4 ����������CO��CO2��CԪ����+2�ۡ�+4�ۣ�ʧ���ӻ��ϼ����ߣ�����CO�ǻ�ԭ����CԪ�ر�������
�ۡ�0�ۣ��õ��ӻ��ϼ۽��ͣ�����Fe3O4 ����������CO��CO2��CԪ����+2�ۡ�+4�ۣ�ʧ���ӻ��ϼ����ߣ�����CO�ǻ�ԭ����CԪ�ر�������

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c2(HI) |
| c(H2)c(I2) |
| c2(HI) |
| c(H2)c(I2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�Ͼ���ʮ���и�һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
 3Fe+4CO2���У�______����������______�ǻ�ԭ����______Ԫ�ر�������
3Fe+4CO2���У�______����������______�ǻ�ԭ����______Ԫ�ر��������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com