
��8�֣�
�����ܡ�G=��H-T��S�жϻ�ѧ��Ӧ���Է��Ը���ѧ����ʽ��TΪ����ѧ�¶ȣ�������㡣������G<0�������Է�����G>0�����̲��Է���������Է�������G=0���ﵽƽ��̬����֪��C��ʯī��S����C�����ʯ��S������H=+1.9 kJ��mol-1����S=��3.4 J��mol-1��K-1���ش��������⣺
��1��ʯī�Ƚ��ʯ ���ȶ����ȶ�����������ʯīת��Ϊ���ʯ�� ���Է�����Է������̡�
��2�����º˱�ը��ʹ�ز��е�̼�����������ʯ���жϺ˱�ը�����ĸ����ܷ�ı�÷�Ӧ���Է��ԣ� �����ݸ���ʵ����������ΪijЩ���ⷴӦ���Է�����ʱ���� �йء�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1 |
| 2 |
| 1 |
| 2 |
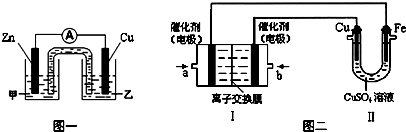
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ������һ�и߶���ѧ����ĩ�������ƻ�ѧ�Ծ����������� ���ͣ������
��8�֣���Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ��
�� 2H2(g)+O2(g)=2H2O(l)  H=��570kJ/mol ���� H2(g)+1/2O2(g)=H2O(g)
H=��570kJ/mol ���� H2(g)+1/2O2(g)=H2O(g)  H="-242kJ/mol"
H="-242kJ/mol"
�� C(s)+1/2O2(g)="CO" (g)  H=" ��110.5kJ/moL" �� C(s)+O2(g)=CO2(g)
H=" ��110.5kJ/moL" �� C(s)+O2(g)=CO2(g)  H="-393.5kJ/moL"
H="-393.5kJ/moL"
�� CO2(g) +2H2O(g)=2CH4(g) +2 O2(g)  H=" +890kJ/moL"
H=" +890kJ/moL"
�ش��������⣺
��1��������Ӧ���������ȷ�Ӧ���У���д��ţ� ��
��2��H2��ȼ����Ϊ ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á� ��֪C(s) + H2O(g) = H2(g)+ CO (g)  H=akJ/moL��
H=akJ/moL��
��a= �� ����֪������ G=
G= H��T
H��T S����
S���� G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�����и߶���ѧ����ĩ�������ƻ�ѧ�Ծ��������棩 ���ͣ������
��8�֣���Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ��
�� 2H2(g)+O2(g)=2H2O(l)
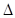 H=��570kJ/mol ���� H2(g)+1/2O2(g)=H2O(g)
H=��570kJ/mol ���� H2(g)+1/2O2(g)=H2O(g)
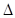 H="-242kJ/mol"
H="-242kJ/mol"
�� C(s)+1/2O2(g)="CO" (g) 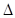 H=" ��110.5kJ/moL" �� C(s)+O2(g)=CO2(g)
H=" ��110.5kJ/moL" �� C(s)+O2(g)=CO2(g)
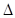 H="-393.5kJ/moL"
H="-393.5kJ/moL"
�� CO2(g) +2H2O(g)=2CH4(g)
+2 O2(g) 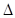 H=" +890kJ/moL"
H=" +890kJ/moL"
�ش��������⣺
��1��������Ӧ���������ȷ�Ӧ���У���д��ţ� ��
��2��H2��ȼ����Ϊ ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á� ��֪C(s)
+ H2O(g) = H2(g)+ CO (g) 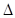 H=akJ/moL��
H=akJ/moL��
��a= ��
����֪������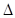 G=
G=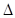 H��T
H��T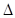 S����
S����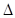 G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ĩ�� ���ͣ������
 H=��570kJ/mol ��
H=��570kJ/mol �� H=-242kJ/mol
H=-242kJ/mol  H= -110.5kJ/moL
H= -110.5kJ/moL  H=-393.5kJ/moL
H=-393.5kJ/moL  H= +890kJ/moL
H= +890kJ/moL  H=akJ/moL����a= �� ����֪������
H=akJ/moL����a= �� ����֪������ G=
G= H-T
H-T S����
S���� G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է����� ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com