�⣺��1��ClO
2��Cl
2�Ļ�ԭ���ﶼΪCl
-��ÿĦ��Cl
2�õ�2mol���ӣ���ÿĦ��ClO
2�õ�5mol���ӣ�������Cl
2�����ʵ�����ClO
2��2.5�����ʴ�Ϊ��2.5��
��2���������ƾ���ǿ�����ԣ�������ɱ����������ԭ����Fe
3+��Fe
3+����ˮ����������������������ˮ�е����ʣ���������ȥˮ�е�������ﵽ��ˮ��Ŀ�ģ�
�ʴ�Ϊ��FeO
42-��ǿ�������ԣ���ɱ����������������ԭΪFe
3+��Fe
3+����ˮ����������������������ˮ�е����ʣ��ﵽ��ˮ��Ŀ�ģ�
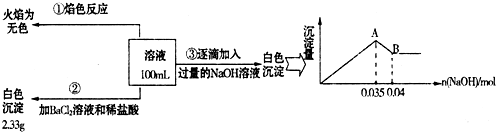
��3������1.0mol/L��NaOH��Һ100mL������ʵ�鲽��������������������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
��ʵ�����A��B������Al��OH��
3��OH
-������Ӧ���䷽��ʽΪ��Al��OH��
3+OH
-=AlO
2-+2H
2O���ʴ�Ϊ��Al��OH��
3+OH
-=AlO
2-+2H
2O��
�۸���ʵ���ȷ����Na
+������ʵ���ȷ����SO
42-������ʵ���ȷ����Al
3+��Fe
2+����ΪCO
32-��Al
3+���ܹ��棬������CO
32-������Һ�д��ڵ�����Ϊ��Al
3+��SO
42-����֪���ᱵ����Ϊ2.33g����n��SO
42-��=
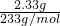
=0.01mol��
����ͼ���֪��Al��OH��
3��OH
-��n��OH
-��=0.005mol��
Al��OH��
3 +OH
-=AlO
2-+2H
2O Fe
2++2OH
-=Fe��OH��
2��
n��Al
3+�� 0.005mol n��Fe
2+�� 0.035-3n��Al
3+��
����n��Al
3+��=0.005mol��n��Fe
2+��=0.01mol ������Һ��Fe
2+��Al
3+�����������SO
42-��������ɲ���ȣ���˴���NO
3-��
��NO
3-���ʵ���Ϊnmol�����ݵ���غ�ã�3n��Al
3+��+2n��Fe
2+��=2n��SO
42-��+n��NO
3-������n��NO
3-��=0.015mol������c��NO
3-���T
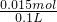
=0.15mol/L��
�ʴ�Ϊ�����ڣ�0.15mol/L��
��������1��ÿĦ��Cl
2�õ�2mol���ӣ���ÿĦ��ClO
2�õ�5mol���ӣ���Ϊ2.5����
��2���������ƾ���ǿ�����ԣ�������ɱ����������ԭ����Fe
3+��Fe
3+����ˮ����������������������ˮ�е����ʣ�
��3������1.0mol/L��NaOH��Һ100mL������ʵ�鲽��������������������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ��
��ʵ�����A��B������Al��OH��
3��OH
-������Ӧ��
�۸���ʵ���ȷ����Na
+������ʵ���ȷ����SO
42-������ʵ���ȷ����Al
3+��Fe
2+����ΪCO
32-��Al
3+���ܹ��棬������CO
32-������Һ�д��ڵ�����Ϊ��Al
3+��SO
42-���������ᱵ�������n��SO
42-��������ͼ�����n��Al
3+����n��Fe
2+�����ٸ��ݵ���غ�ȷ����û��NO
3-��
���������⿼���˳������Ӽ��飬������жϺ����Ӽ��飬�ؼ���ͼ������������������ӹ�����жϣ���Ŀ�Ѷ��еȣ�

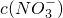 =______���������ڣ����ʲ������𣩣�
=______���������ڣ����ʲ������𣩣�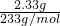 =0.01mol��
=0.01mol��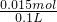 =0.15mol/L��
=0.15mol/L��