
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�
��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�
��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�
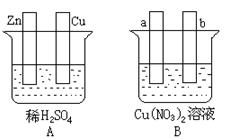
��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ�
�����Ӻ��װ�ý� ��
�缫��Ӧʽ��п�壺 _______________��
ͭ�壺 ___________
��2����Bͼ�У���a��b��Ϊ���Ե缫��
ʹa������ͭ������Ա�Ҫ�����Ӻ�װ�ý� ��
�缫��Ӧʽ��a���� ��b���� ���� ��
�ھ���һ��ʱ���ֹͣ��Ӧ��������Һ����Һ��pH �����ߡ����͡����䣩������һ������ �������� ����Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ɽһ�и߶��ڶ��ε��п��Ի�ѧ�Ծ����������� ���ͣ������
��9�֣���Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�
��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ������Ӻ��װ�ý� ���缫��Ӧʽ��
п�壺 ��ͭ�壺 ��
��2����Bͼ�У�a�� b����ʯī�缫��ʹa������ͭ����b������ �����Ա�Ҫ�����Ӻ缫��Ӧʽ��a���� b���� ������һ��ʱ���ֹͣ��Ӧ�����һ������ ����Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶��ڶ��ε��п��Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣���Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�

��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ������Ӻ��װ�ý� ���缫��Ӧʽ��
п�壺 ��ͭ�壺 ��
��2����Bͼ�У�a�� b����ʯī�缫��ʹa������ͭ����b������ �����Ա�Ҫ�����Ӻ缫��Ӧʽ��a���� b���� ������һ��ʱ���ֹͣ��Ӧ�����һ������ ����Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com