
�ڱ�״���£���aLNH3��ȫ����ˮ�õ�VmL��ˮ����Һ���ܶ�Ϊ��g��cm��3�����ʵ���������Ϊ�أ����ʵ����ʵ���Ũ��ΪC mol/L��������������ȷ���ǣ� ��
�� �أ� �� C��
�� C��
�� ������Һ���ټ���VmLˮ��������Һ��������������0��5��
�� ������Һ���ټ���1��5VmLͬŨ��ϡ���ᣬ��ַ�Ӧ����Һ������Ũ�ȴ�С��ϵΪ��
C��Cl������C��NH4+����C��H+����C��OH����
A���٢� B���ڢ� C���٢� D���ڢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?����һģ����һ��������Mg��Al�Ļ����Ͷ��1mol?L-1500mLϡ�����У�����ȫ���ܽⲢ�������壮����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������
��2009?����һģ����һ��������Mg��Al�Ļ����Ͷ��1mol?L-1500mLϡ�����У�����ȫ���ܽⲢ�������壮����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ��������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a |
| 11.2 |
| a |
| 11.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
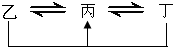 Ԫ��A��B��C��D��E��F��λ��Ԫ�����ڱ�ǰ������Ԫ�أ���ԭ������������������ D��FΪ��������Ԫ�أ�AԪ��ԭ�Ӻ���ֻ��һ�����ӣ�Ԫ��A��B�γɵ���̬��������ڱ�״���µ��ܶ�Ϊ0.759g?L-1��CԪ��ԭ�ӵ�����������������Ӳ�����3����E��Cͬ���壬��D��FԪ����ɵĵ��ʻ�ijЩ���ӣ��������Һ�о�������ת����ϵ���������뷴Ӧ������δ�г��������У�����DԪ�ص��ҡ������������ת��ȫΪ��������ԭ��Ӧ������FԪ�ص��ҡ������������ת��ȫΪ������ԭ��Ӧ�����ڵ����������붡�����������������ѧ��Ӧ����ش��������⣺
Ԫ��A��B��C��D��E��F��λ��Ԫ�����ڱ�ǰ������Ԫ�أ���ԭ������������������ D��FΪ��������Ԫ�أ�AԪ��ԭ�Ӻ���ֻ��һ�����ӣ�Ԫ��A��B�γɵ���̬��������ڱ�״���µ��ܶ�Ϊ0.759g?L-1��CԪ��ԭ�ӵ�����������������Ӳ�����3����E��Cͬ���壬��D��FԪ����ɵĵ��ʻ�ijЩ���ӣ��������Һ�о�������ת����ϵ���������뷴Ӧ������δ�г��������У�����DԪ�ص��ҡ������������ת��ȫΪ��������ԭ��Ӧ������FԪ�ص��ҡ������������ת��ȫΪ������ԭ��Ӧ�����ڵ����������붡�����������������ѧ��Ӧ����ش��������⣺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2006��2007ѧ�����ѧ�ڸ����¿��Ծ�(��)����ѧ���� ���ͣ�013
|
�ڱ�״���£���30gMg��Al��Fe��Zn�ķ�ĩ��ɵĽ����������뵽�����������У���ȫ��Ӧ�ɴ���Һ�еõ�78g�����ξ���(�����ᾧˮ)���������ɵ��������Ϊ | |
| [����] | |
A�� |
5.6L |
B�� |
11.2L |
C�� |
22.4L |
D�� |
33.6L |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com