
[s1] ��t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12,��
��1�����¶��£�ˮ�����ӻ�����kw= ��
��2���ڸ��¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ���Һ��pH== ��
��3�����¶��£���100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ�� ��
��4�����¶��£�pH=2��ij��HA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���Է�����ԭ�� ��
[s1]28��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����һģ����������β���Ǽ��ٳ��п�����Ⱦ���ȵ��о����⣮
��2013?����һģ����������β���Ǽ��ٳ��п�����Ⱦ���ȵ��о����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��֣��ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
����β���dz��е���Ҫ������Ⱦ��о���������β����Ϊ������������Ҫ����
��1��������ȼ������ʱ������Ӧ��N2(g) + O2(g)  2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol��
�� 5 min�ڸ÷�Ӧ��ƽ��������(NO) = ����T ��ʱ���÷�Ӧ��ƽ�ⳣ��K = ��
�� ��Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯���� ������ţ���
a. ���������ܶ� b. ��������ѹǿ
c. ����Ӧ���� d. ��λʱ���ڣ�N2��NO��������֮��
��2��H2��CO���Դ���ԭNO�Դﵽ������Ⱦ��Ŀ�ġ�
��֪��N2(g) + O2(g) = 2NO(g) ?H =" +180.5" kJ��mol��1
2H2(g) + O2(g) = 2H2O(l) ?H = ��571.6 kJ��mol��1
��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ�� ��
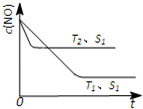
��3��������һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ��ʾ��������������ʱ����Ӧ��2NO(g) + 2CO(g) 2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
�� �÷�Ӧ��?H 0 ���������������
�� �������ı����S1��S2 ������ͼ�л���c(NO) ��T1�� S2 �����´ﵽƽ������еı仯���ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
��������β���Ǽ��ٳ��п�����Ⱦ���ȵ��о����⡣
��1��������ȼ������ʱ�����ķ�ӦN2(g) + O2(g) 2NO(g)�����ɵ�NO������β������Ҫ��Ⱦ�T ��ʱ����5L�ܱ������г���6.5 mol N2��7.5 mol O2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol�������Ǻ�����Ӧ������
2NO(g)�����ɵ�NO������β������Ҫ��Ⱦ�T ��ʱ����5L�ܱ������г���6.5 mol N2��7.5 mol O2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol�������Ǻ�����Ӧ������
��5 min�ڸ÷�Ӧ��ƽ�����ʦ�(NO) = ����T ��ʱ���÷�Ӧ��ƽ�ⳣ��K = ��
�� ��Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯���� ������ţ���
a�����������ܶ� b����������ѹǿ
c������Ӧ���� d����λʱ���ڣ�N2��NO��������֮��
��2����H2��CO����ԭNO���Դﵽ������Ⱦ��Ŀ�ġ�
��֪��2NO(g) = N2(g) + O2(g) ��H = ��180.5 kJ��mol��1
2H2O(l) =2H2(g) + O2(g) ��H = +571.6 kJ��mol��1
��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ��
��
��3��������һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ��ʾ��������������ʱ����Ӧ2NO(g) + 2CO(g) 
 2CO2(g)
+ N2(g) �У�NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
2CO2(g)
+ N2(g) �У�NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�

�� �÷�Ӧ��H 0 ���������������
�� �������ı����S1��S2 ������ͼ�л���c(NO) ��T1�� S2 �����´ﵽƽ������еı仯���ߣ�������Ӧ��ע����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[s1] ��t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12,��
��1�����¶��£�ˮ�����ӻ�����kw= ��
��2���ڸ��¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ���Һ��pH== ��
��3�����¶��£���100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ�� ��
��4�����¶��£�pH=2��ij��HA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���Է�����ԭ�� ��
[s1]28��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com