
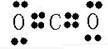
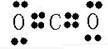
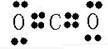 £¨
£¨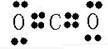 £ª
£ª

 ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏
ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏ –°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
–°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
»ð∆˜Œ¬∂» ≈®∂» |
a»ð∆˜ 400°Ê |
b»ð∆˜ 425°Ê |
c»ð∆˜ 450°Ê |
d»ð∆˜ 475°Ê |
e»ð∆˜ 500°Ê |
| c£®O2£© | 0.8 | 0.6 | 0.3 | 0.5 | 0.7 |
| c£®SO3£© | 0.4 | 0.8 | 1.4 | 1.0 | 0.6 |

| c2(SO3) |
| c2(SO2)°¡c(O2) |
| c2(SO3) |
| c2(SO2)°¡c(O2) |


≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫



| ¢ŸO3 | ¢⁄Zn£¨H2O |




≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
 ‘™Àÿ÷Ð∆⁄±Ì∂Ã÷Ð∆⁄µƒ“ª≤ø∑÷»Á±ÌÀ˘ æ£Æœ¬¡–”–πÿA°¢B°¢C°¢D°¢EŒÂ÷÷‘™Àÿµƒ– ˆ÷–£¨’˝»∑µƒ «£®°°°°£©
‘™Àÿ÷Ð∆⁄±Ì∂Ã÷Ð∆⁄µƒ“ª≤ø∑÷»Á±ÌÀ˘ æ£Æœ¬¡–”–πÿA°¢B°¢C°¢D°¢EŒÂ÷÷‘™Àÿµƒ– ˆ÷–£¨’˝»∑µƒ «£®°°°°£©≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com