
| A��֤�������е���������������ԭ��Ӧ��ʵ�飨�������⣩�� | B���ܽ���ЧӦʵ�飨�����������ˮ���� | C��װ�õ������Լ���ʵ�飻 | D������ijЩ�������ʵ�ʵ�飨��CO2��SO2��Cl2�������Һ�ķ�Ӧ���ȵȡ� |
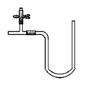
 ���ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����
���ƿ��С�Թܡ���ͷ�ιܡ���Ƥ�����齺�ܡ����ܣ���ʵ��ҩƷ���Լ��Զ���������ͼ��ע����
 �����ƿ����������;�治��ʵ������Zn�������ᷴӦ�Ƶõ�H2�����к�������ˮ�������⼰�����������������壬ijͬѧ������ֻ���ƿ��������¼���װ�ã���һ��˳�����ӣ���ﵽ�˵�����ͨ��ʱ��ÿһװ�ó�ȥһ�������Ŀ�ġ�
�����ƿ����������;�治��ʵ������Zn�������ᷴӦ�Ƶõ�H2�����к�������ˮ�������⼰�����������������壬ijͬѧ������ֻ���ƿ��������¼���װ�ã���һ��˳�����ӣ���ﵽ�˵�����ͨ��ʱ��ÿһװ�ó�ȥһ�������Ŀ�ġ�
 2�����ӷ���ʽΪ________________���۲쵽������Ϊ________________��
2�����ӷ���ʽΪ________________���۲쵽������Ϊ________________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
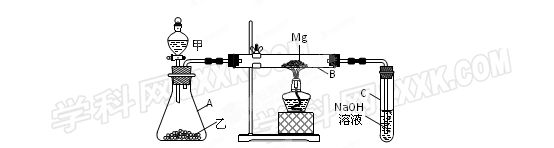 ��1��ѡ����ȡSO2��������Լ� ������ţ���
��1��ѡ����ȡSO2��������Լ� ������ţ���
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������������Һ��ɼ����������ƺ�ʳ�� |
| B����ѹ������Ϊ�˼ӿ�����ٶȣ��õ��ϴ�����ľ��� |
| C����������茶�����˺�����ˮ�Ҵ�ϴ�� |
| D��ֽ���������������Ӻ�ͭ����ʵ���У�չ�����ijɷ�Ϊ����ˮ���ͪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ɫ�����������������ʹ����ʯ��ˮ����ǣ�ԭ��Һ��һ����CO32�� |
| B�����Ȼ�����Һ�а�ɫ�����������ټ����ᣬ��������ʧ��ԭ��Һ��һ����SO42�� |
| C��������������Һ���Ȳ���������ʹʪ���ɫʯ����ֽ������ԭ��Һ��һ����NH4�� |
| D������NaOH����Һ���Ȳ�����ɫ����������ʧ��ԭ��Һ��һ����Mg2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
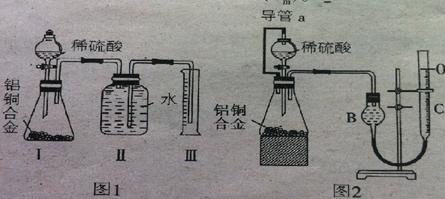
| | ʵ��ǰ | ʵ��� |
| ��ͭ�Ͻ�������g�� | m1 | m2 |
| ��Һ�ܣ�C�������mL�� | V1 | V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ñ�����KMnO4��Һ�ζ�Na2SO3��Һ�Բ�����Ũ�ȣ�KMnO4�����Ϻ�ɫ |
| B�����á�Ag����SCN��===AgSCN����ԭ�������ñ�KSCN��Һ����AgNO3��ҺŨ�ȣ�Fe(NO3)3������ɫ |
C�����á�2Fe3����2I��===I2��2Fe2������ ��FeCl3��Һ����KI��Ʒ��KI�İٷֺ����� ��FeCl3��Һ����KI��Ʒ��KI�İٷֺ��������ۡ�����ɫ |
| D������OH����H��===H2O������ij������Һ��Ũ��ʱ����̪����dz��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��AlN���Ʊ���Al2O3+N2+3C
��AlN���Ʊ���Al2O3+N2+3C 2AlN+3CO����Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ ��
2AlN+3CO����Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ �� CO2+2H2O+4Cu��Ϊ�ⶨ��Ʒ���йسɷֵĺ����������������£�
CO2+2H2O+4Cu��Ϊ�ⶨ��Ʒ���йسɷֵĺ����������������£�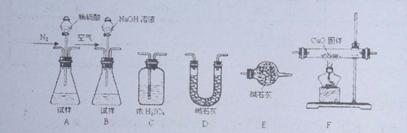
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���ռ����� | B��ת����Һ | C��ģ�ҵ�Ʊ������鰱�� | D���ú�ˮ����������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢۢ� | C���ڢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com