
ijУ��ѧС���ͬѧ��չ��һϵ�еĻ�ѧʵ����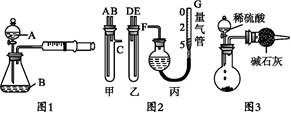
�������ʵ�鲢�����������:
(1)��ͬѧ��ͼ1��ʾװ��,����п�����ᷴӦ����:��2 gп��������ƿ��,ͨ����Һ©������1 mol��L-1ϡ����40 mL,�ռ�10 mL����,ͨ����¼�������������õ���Ӧ����Ϊx mol��(L��min)-1��ʵ�鿪ʼʱ����װ�������Եķ�������������������������������
(2)��ͬѧ��ͼ2װ�òⶨNa2CO3��NaCl�Ĺ���������Na2CO3����������:
�ټס������Թܸ�����������,�������Ӷ�Ӧ�ӿں�,����ʢϡ������Թ�,������Ӧ,�ų�����,����������ϡ����Ӧ�ֱ�����������������������(���������);
��G�ܿ����û�ѧʵ�������һ�ֳ������������,����������������;
�������ס��ҽӿڵ����ӷ�ʽ����:A��������,B����������,C����������(��д���ӿڵı��);
��Ϊ��߲�����ȷ��,�ռ��������,��װ�ö���ǰӦ���еIJ�����������������������������������������������������
(3)��ͬѧ���ͬѧʵ��Ŀ����ͬ:��ͼ3װ�òⶨ���ɵ�CO2������,����װ�ô�������ȱ��,�Ӷ�����ʵ�����,�����������ʹ�ⶨ�������ƫ�����Ҫԭ��������������������������������
 ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С����������в��ἰ�����εĺ���(��C2O42-��)��ʵ�鲽�����£�
�ٽ�������ƷԤ��������ˮ���ݣ����˵õ����в��ἰ�����ε���Һ��
�ڵ�����Һ������ԣ��μ�����CaCl2��Һ��������ɫ�����������������ᣬʹCaCO3�ܽ⣻���˵õ�CaC2O4���塣
����ϡHCl�ܽ�CaC2O4����ˮ����100 mL��Һ��ÿ��ȷ��ȡ25.00 mL����Һ����0.0100 mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��
�ش��������⣺
(1)������С���ƷԤ�������ķ����� (A.���ճɻң�B.��ĥ��֭)��
(2)������С�������Һ������ԡ��� (A.�����ԣ�B.�����ԣ�C.����)����֤CaCl2��Һ�ѡ��������IJ����������� ��
(3)��������õ��IJ����������ձ�����ƿ����ͷ�ιܡ��������⣬���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32��+ I2 �� S4O62��+ 2I-
38.����100 mL0.0500 mol/L I2��Һ������Ҫ�������� ��ѡ���ţ���
a��100 mL����ƿ b����Ͳ c���ձ� d��������
�ζ��ܱ���ʹ���¶ȣ�20oC; �ζ��ܵ���С�̶�Ϊ mL��
39.ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500 mol/L I2��Һ�ζ���ʵ����������(��3�γ�����Ϊ 0.00���յ������ͼ; ���ʲ��μӷ�Ӧ)��
| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ�����Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��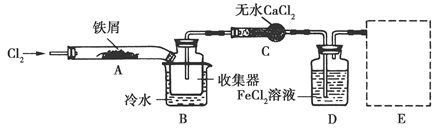
��.���������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ��ͼ(���ȼ��г�װ����ȥ)�������������£�
�ټ���װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ��ɣ�
�ܡ���
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����N2�Ͼ�Cl2�����ռ����ܷ⡣
��ش��������⣺
(1)װ��A�з�Ӧ�Ļ�ѧ����ʽΪ______________��
(2)�ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A�Ҷˡ�Ҫʹ������FeCl3�����ռ������ڢܲ�������________��
(3)���������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ��(������)________��
(4)װ��B����ˮԡ������Ϊ________��װ��C������Ϊ________��װ��D��FeCl2ȫ����Ӧ����ʧȥ����Cl2�����ö�ʧЧ��д������FeCl2�Ƿ�ʧЧ���Լ���________��
(5)�����߿��л���β������װ��E��ע���Լ���
��.����ͬѧ��װ��D�еĸ���ƷFeCl3��Һ����H2S���õ��������˺�����ʯīΪ�缫����һ�������µ����Һ��
(6)FeCl3��H2S��Ӧ�����ӷ���ʽΪ________________��
(7)������H���������ŵ����H2�������ĵ缫��ӦʽΪ____________________��
(8)�ۺϷ���ʵ����������Ӧ����֪��ʵ�������������ŵ㣺
��H2S��ԭ��������Ϊ100%����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ͭ�����ڲ�ͬ�¶��¿�ʧȥ���ֻ�ȫ���ᾧˮ�����ֽ�������ijѧ���ڲ�ͬ�¶��¸�8.000 g����ͭ������ȣ��¶������ߣ���ʵ������¼���£�
| ʵ����� | �¶ȣ��棩 | ��ȴ��ʣ������������g�� |
| 1 | 102 | 6.848 |
| 2 | 113 | 5.696 |
| 3 | 258 | 5.120 |
| 4 | 570 | 2.560 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��������ҹ���Ҫ�ĵ���Ʒ��֮һ���������������������ӷ���ʧ��Ϊ�˼�����������ȷ�����ʩ����������ⶨ�京������
��ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�

��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ ��
��2����Һ©���е�Һ�����ʺϵ��� ��
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D���������� |
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1��20 | 16��21 |
| 2 | 3��00 | 18��90 |
| 3 | 4��50 | 19��49 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ��Ƴ����ڵ�·�ڱ��������������������ˮ�������������ʡ�ʵ�����ù�ҵ����ʯ����������Al2O3��Fe2O3�����ʣ��Ʊ��Ȼ��Ƶ���Ҫ�������£� ���������գ�
���������գ�
��1������ʹ�õ���������ʵ���Ũ��ԼΪ6.0mol/L������36.5%�����ᣨ�ܶ�Ϊ1.2g/mL������6.0mol/L������100mL������IJ��������в���������Ͳ����ͷ�ιܡ� ����Ҫ��ȡ36.5%������ mL�����ƹ����У���������������ȷ�����в���������Ũ��ƫС���� ��
| A������ҡ�Ⱥ���Һ����ڿ̶��� |
| B������ʱ��������ƿ�Ŀ̶��� |
| C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ��� |
| D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�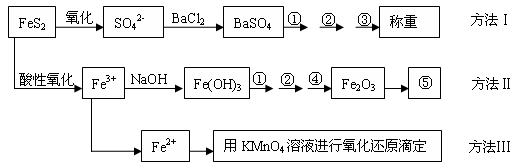
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ͼ���Ļ������ü������ڽ�Լ��Դ���������ڱ���������ij�о�С��ͬѧ�ԷϾ�п�̸ɵ��Ϊԭ�ϣ����Ͼɵ�غ�п����ת����ZnSO4��7H2O�����̲���ת���ɴ��Ƚϸߵ�MnO2����NH4Cl��ҺӦ���ڻ��������У�ʵ���������£�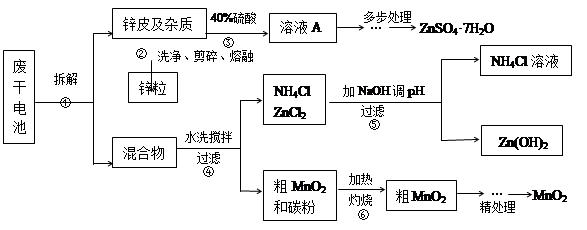
��1�������������õļ�������Ӧѡ ��ѡ�����������������
��2������ҺA�����ĵ�һ���Ǽ��백ˮ����pHΪ9��ʹ���е�Fe3+��Zn2+��������д����ˮ��Fe3+��Ӧ�����ӷ���ʽ ��
��3����������Ϊ�˳�ȥ��Һ�е�Zn2+����֪25��ʱ��
| NH3��H2O��Kb | Zn2+��ȫ������pH | Zn(OH)2���ڼ��pH |
| 1.8��10��5 | 8.9 | ��11 |
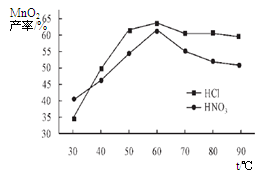
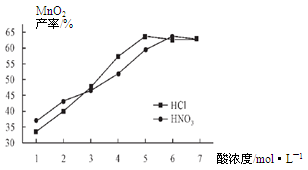
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com