ij��±�����ķ�Һ�к��д�����K
+��Cl
-��Br
-����������Ca
2+��Mg
2+��SO
42-��ij�о���ѧϰС����ȡ�÷�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ�壨Br
2��������������������̣�
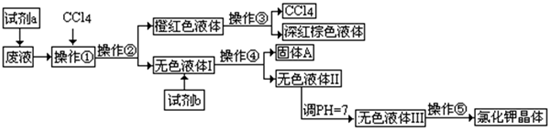
���пɹ�ѡ���a��b�Լ�������Na
2CO
3��Һ������K
2CO
3��Һ��KOH��Һ��BaCl
2��Һ��Ba��NO
3��
2��Һ��H
2O
2��KMnO
4��H
+����Һ��ϡHNO
3��������������̣��ش�������⣺
��1���Լ�aӦѡ
H2O2
H2O2
���÷�Ӧ�����Ի�������ɣ������ӷ���ʽΪ
H2O2��H2O2+2Br-+2H+=Br2+2H2O
H2O2��H2O2+2Br-+2H+=Br2+2H2O
��
��2�������١��ڡ��ۡ��ܡ��ݵ���������Ϊ
D
D
��ѡ����ĸ����
A����ȡ�����ˡ���Һ�����ˡ������ᾧ B����ȡ����Һ����Һ�����ˡ������ᾧ
C����Һ����ȡ�����ˡ����ˡ������ᾧ D����ȡ����Һ�������ˡ������ᾧ
��3�����������õ��Ĵ�������������
������
������
��
��4��ѡ����ȥ��ɫҺ��I��Ca
2+��Mg
2+��SO
42-����������Լ������μ�˳������Ϊ
BaCl2��KOH��K2CO3
BaCl2��KOH��K2CO3
���ѧʽ����
��5������pH��������
��ȥ������OH-��CO32-
��ȥ������OH-��CO32-
��




 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�