
��4�֣�ʵ������Ҫ0.1mol/L����950ml��ijͬѧ��98%��Ũ���ᣨ�ܶ�Ϊ1.84g��cm-3�����ƣ�
��1����ѡ�� ����ƿ��
A��50m B��100ml C��250ml D��1000ml
��2������ȡŨ���� ml��
��3��������Һʱ���в���ʹ������ҺŨ��ƫ����� ��
A������ƿ��ԭ����������ˮ B��ϴ���ձ��Ͳ���������Һδת������ƿ��
C������ʱ�۲�Һ�温�� D����Һδ����ȴ��ע������ƿ
��4��������Һʱ�����¼���������
���ܽ� ��ҡ�� ��ϴ�� ����ȴ ����ȡ ��ת����Һ �߶���
��ȷ�IJ���˳���� ������ţ���
��4�֣���1�� D ��2��5.4ml ��3��C D ��4�� �ݢ٢ܢޢۢߢ�
��������
�����������1��Ӧ������ƿ�Ĺ��û��950ml�ģ�����Ӧ������1000ml�ģ���ѡD��
��2����ҪŨ����������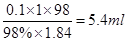 ��
��
��3������c��n/V��֪���������ƿ��ԭ����������ˮ����Ӱ��ʵ������ϴ���ձ��Ͳ���������Һδת������ƿ�У������ʼ��٣�Ũ�Ƚ��ͣ�����ʱ�۲�Һ�温�ӣ�������ƿ����Һ��������٣�Ũ��ƫ�ߣ���Һδ����ȴ��ע������ƿ�����������������֪������ƿ����Һ��������٣�Ũ��ƫ�ߣ���ѡCD��
��4���������Ƶ�ԭ����ʵ��Ҫ���֪����ȷ�IJ���˳���Ǣݢ٢ܢޢۢߢڡ�
���㣺�������ʵ���Ũ�ȵ����ơ���������
����������һ�����ʵ���Ũ����Һ��ʵ������ѧ��ѧ��һ����Ҫ�Ķ���ʵ�飬ʵ�������������ҺŨ�ȴ������������кܶࡣ�Ӵ�ķ��潲��һ����ʵ������еIJ��淶��������ģ�������������ҩƷ��ϵͳԭ������ġ�������������ԭ���ӣ������������ͳ�Ϊ�߿���ѧʵ���е�һ���ѵ㣬��Ҫע���ܽ�ͻ��ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺
��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com