
�����15�֣�
��4�������ȿ�����ͬ��ͬ��þ���Ͻ���Ʒ�١��ڡ��ۡ��ܡ��ס��ҡ�������4λͬѧ��ȡ1����Ʒ����������ʵ�飬�ⶨ�Ͻ���þ������������
52����ͬѧȡ��Ʒ��m1 g����������������Һ��Ӧ��Ȼ����ˣ�������Һ��ͨ������Ķ�����̼���壬�����ó������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm1 g������Ʒ�ٺϽ���þ����������Ϊ_____________��
53����ͬѧȡ��Ʒ��m2 g�����������ᷴӦ��Ȼ��μӹ���������������Һ�����������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm2 g������Ʒ�ںϽ���þ����������Ϊ____________��
54����ͬѧȡ��Ʒ��m3 g��������ϡ���ᷴӦ�����ֹ�����ȫ�ܽ⣬��״���µõ��������ΪV L������Ʒ����m3��ȡֵ��Χ��___________________________________________��
��ͬѧȡ��ͬ��������Ʒ�ֱܷ��30 mLͬŨ�ȵ����ᷴӦ����ȡ�Ͻ���������������������ת��Ϊ��״�������£�
| ʵ����� | a | b | c |
| �Ͻ�����/mg | 510 | 765 | 918 |
| �������/mL | 560 | 672 | 672 |
52. 47.06%������2�֣�
53. 60%������2�֣�
54. 0.80V��m��1.07V
��2�֣�����д����27V/33.6��m��24V/22.4��9V/11.2��m��3V/2.8�����֣�
55. �������ᷴӦ��ȫ��n(H2)="0.672/22.4" ="0.03" mol����1�֣�
��c(HCl)="0.03��2/0.03=2.0" mol��L-1��1�֣�
56. ��Ͻ���Mg��Al�����ʵ����ֱ�Ϊx��y�����У�
��1�֣�
��ã�x =" y" =" 0.01" mol��1�֣�
��w(Mg)="[(0.01" mol��24 g��mol-1)/0.510 g]��100%=47.06%��1�֣�
57. ����HCl ~ NaCl��Al ~ NaAlO2��֪
n(NaCl)="n(HCl)=2.0" mol��L-1��0.030 L="0.06" mol��1�֣�
n(NaAlO2)="n(Al)=0.01" mol��918/510="0.018" mol��1�֣�
����Na+�غ�ã�n(NaOH)="0.06" mol+0.018 mol="0.078" mol��1�֣�
��V(NaOH)="0.078" mol/1.0 mol��L-1="0.078" L="78" mL ��1�֣�
��c��ʵ��������78 mL������������Һ������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⡣
�������������52.þ���Ͻ��������������Ʒ�Ӧ���ᆳ��������̼��Ӧ���ó������ˡ�ϴ�ӡ���ɡ����գ��õ�����Ϊ����������ӦǰΪþ����Ӧ��Ϊ���������������䣬����Ԫ���غ��֪����������������������Ϊþ������������Ϊ16��3/��16��3+27��2��= 47.06%��53.�÷�Ӧ�õ��Ĺ���Ϊ����þ������Ԫ�������غ㣬����þ��þԪ�ص�������������þ���Ͻ���þԪ�ص�����������Ϊ24/��24+16��= 60%��
54.þ����ϡ���ᷴӦ���ĵ����ͬ������������Ҳ��ͬ���ʲ��ü�ֵ��ȷ���䷶Χ������ȫΪþʱ
��������ΪV /22.4��24=1.07V������ȫΪ��ʱV /22.4��2/3��27=0.80V,��Χ0.80V��m��1.07V��
55.��ͬѧʵ���������������̶���bc�����ᷴӦ��ȫ��a�������Ӧ��ȫ���ʼ��������Ũ�ȸ���bc����������ֱ�ӵó����������ᷴӦ��ȫ��n(H2)="0.672/22.4" ="0.03" mol��
��c(HCl)="0.03��2/0.03=2.0" mol��L-1
56.����a�������з���ʽ�ɽ⣬�ⷨ���𰸣�57.����Ԫ���غ㣬�Ͻ��������ձ�ΪNaAlO2����ʼ������������ձ�Ϊ�Ȼ��ƣ��˿ɳ�Ϊ��̬��������
n(NaCl)="n(HCl)=2.0" mol��L-1��0.030 L="0.06" mol����ԭ���غ㣩
n(NaAlO2)="n(Al)=0.01" mol��918/510="0.018" mol����ԭ���غ㣩
����Na+�غ�ã�n(NaOH)="0.06" mol+0.018 mol="0.078" mol
��V(NaOH)="0.078" mol/1.0 mol��L-1="0.078" L="78" mL ��1�֣�
��c��ʵ��������78 mL������������Һ������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⡣
���㣺���黯ѧ�����й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������A�����ᷴӦ����dz��ɫ��ҺB��ͬʱ�ų�����C�������B��Һ��ͨ����������Bת����ػ�ɫ��ҺD������ҺD��Ϊ���ݣ�һ�ݼ��뼸��KSCN��Һ����Һ���Ѫ��ɫ����һ�ݼ���A�����ػ�ɫ��ҺD���±��dz��ɫ��ҺB����
��1�������ʵĻ�ѧʽ�ֱ��ǣ�A________ ��B________ ��C________ ��D________��
��2���йط�Ӧ�����ӷ���ʽ�У�
�� B��D___________________________��
�� D��B____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ľ���֮һ��
��1��Ũ���ᡢŨ���������������������ԭ���� ��
��2����������ҪӦ���ڻ���ƽ�������ҵ��������ˮ�Ȼ������⻯��ﮣ�LiAlH4�����л��ܼ��з�Ӧ�Ƶ�����������ѧ����ʽ���£�3LiAlH4+AlCl3="4Al" + 3LiCl + 6H2��
�÷�Ӧ��������Ϊ ��
��3���⻯���ƣ�NaAlH4����һ����Ҫ�Ĵ�����ϣ���֪��
NaAlH4(s)=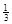 Na3AlH6 (s)+
Na3AlH6 (s)+ 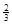 Al (s) + H2(g) ��H��+ 37 kJ��mol��1
Al (s) + H2(g) ��H��+ 37 kJ��mol��1
Na3AlH6(s)="3NaH(s)+" Al (s) + 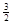 H2(g) ��H��+ 70.5 kJ��mol��1
H2(g) ��H��+ 70.5 kJ��mol��1
��NaAlH4(s)=" NaH(s)" + Al (s) +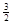 H2(g) ��H�� ��
H2(g) ��H�� ��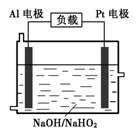
��4����֪H2O2��һ�����ᣬ��ǿ������Һ����Ҫ��HO2����ʽ ���ڡ�Ŀǰ�о��Ƚ����ŵ�Al��H2O2ȼ�ϵ�أ���ԭ������ͼ��ʾ������ܷ�Ӧ���£�
2Al+3HO2��+3H2O =2[Al(OH) 4]��+OH��
��������ӦʽΪ ��
������ͨп�̸ɵ����ȣ���������ͬ�����ĸ�����������ʱ��Al��H2O2ȼ�ϵ�ص����۷ŵ���ԼΪ��ͨп�̸ɵ�ص�______����
��Al�缫�ױ�NaOH��Һ��ѧ��ʴ�����Ǹõ��Ŀǰδ���ƹ�ʹ�õ�ԭ��֮һ����Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ҵ���Ի�ͭ����Ҫ�ɷ�CuFeS2��Ϊԭ���Ʊ�����ͭ�����������ֹ��ա�
I.���������գ����������Ļ�ͭ�����ʯӢ����ͨ�˿������б��գ������Ƶô�ͭ��
��1�����յ��ܷ�Ӧʽ�ɱ�ʾΪ2CuFeS2+ 2SiO2+5O2��2Cu+2FeSiO3+4SO2�÷�Ӧ���������� ��
��2�����д���SO2�ķ���������������_____
A�߿��ŷ� B�ô�����Һ�����Ʊ���������
C�ð�ˮ���պ��پ������Ʊ������ D��BaCl2��Һ�����Ʊ�BaSO3
��3��¯����Ҫ�ɷ���FeO��Fe2O3��SiO2��Al2O3�ȣ�Ϊ�õ�Fe2O3�������ܽ�������������У�δ�漰���IJ����� ��
A���� B�ӹ���NaOH��Һ C�����ᾧ D���� E��������
II. FeCl3��Һ��ȡ������������������ͼ��ʾ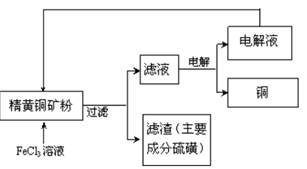
��4�����������У�CuFeS2��FeCl3��Һ��Ӧ�����ӷ���ʽΪ_____________��
��5���ù��������У�����ѭ�����õ������� ���ѧʽ����
��6������ʯī�缫�����Һ��д�������ĵ缫��ʽ_____________��
��7����ͭ���к�����Pb������C1һŨ�ȿɿ�����Һ��Pb2+��Ũ�ȣ���c(C1һ)��2mo1��L-1ʱ����Һ��Pb2+���ʵ���Ũ��Ϊ mol��L-1��[��֪KSP(PbCl2)��1 x 10һ5]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������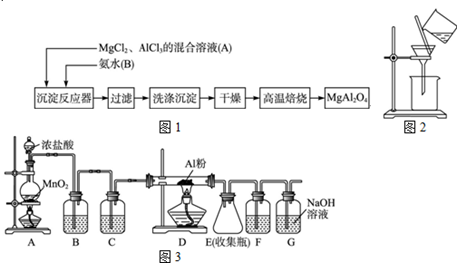
��1��ΪʹMg2+��Al3+ͬʱ���ɳ�����Ӧ���������Ӧ���м��� ���A����B�����ٵμ���һ��Ӧ��Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ ��
��2������ͼ��ʾ�����˲����е�һ�������� ��
��3���ж������г����Ƿ�ϴ�����õ��Լ��� �����±���ʱ������ʢ�Ź�������������� ��
��4����ˮAlCl3��183������������ʪ��������������������ʵ���ҿ�������װ���Ʊ���װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ������ ��F���Լ��������� ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��10�֣���54.4 g���ۺ��������Ļ�����м���200 mL��ϡ���ᣬǡ����ȫ��Ӧ���ų�����4.48 L����״��������Ӧ�����Һ�еμ�KSCN���Ժ�ɫ��������ʣ����ţ�������������������Ƕ��ٿˣ��ƣ�ԭϡ�������ʵ���Ũ�ȣ��ǣ���Ӧ��õ�FeSO4�����ʵ����Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���ǻ��õļ����Ԫ�أ��Ƽ��仯�������������������й㷺��Ӧ�á�
������м��㣺
��1���������ƣ�NaN3����ײ���ֽ�����ƺ͵������ʿ�Ӧ����������ȫ���ҡ���78�˵���������ȫ�ֽ⣬������״���µ���___________________L ��
��2����-�غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05 g��-�غϽ�����200 mLˮ����0.075 mol������������Һ���������Ƶ����ʵ���Ũ��______________________������Һ������仯����
��3������������Һ�����������ˣ��õ��������Ƶ���Һ�������Һ��ͨ�������̼�������з�Ӧ�� 2NaAl(OH)4+CO2��2Al(OH)3��+Na2CO3+H2O����֪ͨ�������̼112 L����״���£������ɵ�Al(OH)3��Na2CO3�����ʵ���֮��Ϊ4:5���������Һ��ͨ��Ķ�����̼Ϊ224L����״���£����������ɵ� Al(OH)3��Na2CO3�����ʵ��������ֵ��
��4��Ϊ�ⶨij�������պ�������������������е�Ԫ�ص������������ֽ���ͬ��������ι���ֱ���뵽50.00mL��ͬŨ�ȵ�����������Һ�У���ˮԡ����������ȫ���ݳ�(���¶�����β��ֽ�)�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ����������ֲⶨ������±���
| ������/g | 10.00 | 20.00 | 30.00 | 50.00 |
| Ũ�������ӵ�����/g | m | m | 1.29 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС���������ռ���������Ϣ���ء��ơ��ơ�þ�Ȼ��ý���������CO2������ȼ�ա����Ƕ�����CO2������ȼ�պ�IJ����еİ�ɫ���ʽ���������̽����
ʵ�飺��ȼ�յ���Ѹ�����뵽ʢ��CO2�ļ���ƿ�У��������м���ȼ�գ���Ӧ����ȴ��ƿ���ź�ɫ������ƿ����𤸽�Ű�ɫ���ʡ�
�������
����1����ɫ������Na2O��
����2����ɫ������Na2CO3��
����3����ɫ������Na2O��Na2CO3�Ļ���
���ʵ�鷽����֤����
��С���ȼ�պ�İ�ɫ����
��������̽����
| ʵ�鷽�� | ʵ����� | ʵ������ | ���� |
| ����1 | ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м�����ɫ��̪��Һ | ��Һ��ɺ�ɫ | ��ɫ����ΪNa2O |
| ����2 | ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м��������CaCl2��Һ | ���ְ�ɫ���� | |
| | �ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
��1�����������õ��IJ����������ձ����������⣬��������________��________(����������)��
��2��д��������ˮ������Ӧ�����ӷ���ʽ_____________________________________��
��3����������Ѿ�ϴ�Ӹɾ��IJ�����������_____________________��
��4����������ΪW1 g�����Ⱥ����������ɫ����������ΪW2 g������Ʒ����Ԫ�ص�����������________ (�г�ԭʼ��ʽ�����軯��)��������ȷ�����ղ����Ľ��ƫ�����������ԭ�������_____________________________________________________________
(д��һ��ԭ��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com