
��ҵ�Ƶõĵ�����(AlN)��Ʒ�г���������Al4C3��Al2O3��C�����ʡ�ijͬѧ���������ʵ��ֱ�ⶨ������(AlN)��Ʒ��AlN��Al4C3����������(����NH3��ǿ������Һ�е��ܽ�)��
(1)ʵ��ԭ��
��Al4C3�����ᷴӦ������CH4;
��AlN����ǿ���������,��������������Һ���ɰ���,��д��AlN��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ����������������������������������
(2)ʵ��װ��(��ͼ��ʾ)
(3)ʵ�����
������ʵ��װ��,����װ�õ������ԡ��Ƶ�Dװ�õ�����Ϊn g,�ζ��ܵĶ���Ϊa mL��
�ڳ�ȡm g AlN��Ʒ������ƿ��;���ý���,�رջ���������,����������,ͨ����Һ©���������������(�ѧʽ),����ƿ�����ʳ�ַ�Ӧ��
�۴���Ӧ������ȫ��,�رջ���������,����������,ͨ����Һ©���������������(�ѧʽ),����ƿ�����ʳ�ַ�Ӧ��
��������������������������(����ò�Ӧ���еIJ���)��
�ݼ�¼�ζ��ܵĶ���Ϊb mL,�Ƶ�Dװ�õ�����Ϊp g��
(4)���ݷ���
��AlN����������������������
������ȡ�ζ�������������ʱ,Һ������ҵ�,��������������������(�ƫ��ƫС������Ӱ�족)��
��Al4C3����������Ϊ��������������(��ʵ�������µ�����Ħ�����ΪVm)
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2012�궬�죬�ҹ����п�����Ⱦ״���ܵ����ǵ�ǿ�ҹ�ע���ڿ������������У�SO2��ָ���Ǻ������������û�����Ҫָ�ꡣΪ�˲ⶨ�����е�SO2����������λͬѧ�ֱ���������²ⶨ������
I��������ԭ������ͬѧ�������ͼ��ʾ��װ�ý���ʵ�飺
��1����ʵ����Ҫ80mL5��10-4mol��L�ĵ���Һ����ͬѧӦ����I2 g����ȷ��0.001����ѡ��_______mL������ƿ�������ơ���ʵ�����漰��������ԭ��Ӧԭ��Ϊ ���û�ѧ����ʽ��ʾ������ʵ��ԭ����������SO2�� �ԡ�
��2���ڼ�ͬѧ�������ҺŨ��ȷ��������ȡҩƷ��ʵ������и��ֶ��������������£���������װ�����ⶨ��SO2������Ȼ��ʵ�ʺ����ͣ���������п��ܵ�ԭ�� .
������������ͬѧ������ͼ ��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��
��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��
��3��ȡ����������SO2����Ϊ mg/L����ȷ��0.001����
��4����֪��������BaSO3��KSPΪ5.48��10-7������������Һ��c��SO2-3��=6��3��10-8mol/L����ͬѧ��Ϊ����ʵ�鲻����H2O2����SO2��ֱ����0��1mol��L BaCl2��Һ������SO2���ɲ��������������������ݷ����������Ƿ���� .
III������������ͬѧֱ��ʹ��һ��SO2Ũ�����ܼ���Dzⶨ�����е�SO2���������ּ���������õ绯ѧԭ�������ݵ�ز���������ǿ����ȷ����SO2Ũ�ȵġ��õ���ܵĻ�ѧ��Ӧԭ��Ϊ��2SO2+O2+2H2O=2H2SO4
��5����д���õ�ظ����ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С�����÷�Ӧ2CuO��2Cl2 2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��
2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��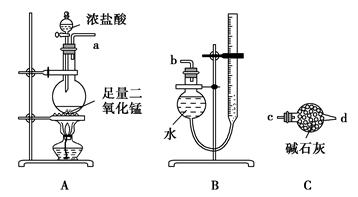

����һ��ͨ���ⶨ��Ӧ��CuO������m(CuO)�Ͳ���O2�����V(O2)���ⶨͭ�Ľ������ԭ��������
(1)����������������ý���(ͼ��δ����)��ѡ���������ϳ�һ��ʵ��װ�ã�����˳��Ϊa��(����)(����)��(����)(����)��(����)(����)��(����)(����)��b��
(2)װ��B���ɸ���ܺͼ�ʽ�ζ��ܸ�����ɵIJ������������װ�ã�ʵ��ǰ�ζ���Һ�������ΪV1 L��ʵ���ָ������£�����װ������Һ����ƽ��õ�ĩ����ΪV2 L��������ʱ����Ħ�����ΪVm L��mol��1����Eװ����CuO������Ϊm1 g����ַ�Ӧ������CuCl2������Ϊm2 g����ͭ�Ľ������ԭ�������ı���ʽΪ
[�ú�m1��V1��V2�Ĵ���ʽ��ʾ]��
(3)������ͭ�л���ͭ����ⶨ��� (�ƫ����ƫС������Ӱ�족)��
(4)װ��E��ʵ������е���Ҫ������ ��
������������A��D��E��F����װ��(β����������װ�ô���)��ɲⶨ����
(5)����Ϊ�ⶨ���������� (д��һ��)������ⶨ����������д��ͭ�Ľ������ԭ�������ı���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС����ʵ������ģ���������̡�����Ƶ�ģ��װ�����£�
����Ҫ����գ�
(1)Bװ�������ֹ��ܣ��پ��Ȼ�����壻�� ���� ��
(2)��V(Cl2)/V(CH4)��x��������������������Ȼ��⣬��xֵӦ ��
(3)Dװ�õ�ʯ���о��Ȼ���KI��ĩ���������� ��
(4)Eװ�õ������� (����)��
A���ռ����� B.��������
C����ֹ���� D�������Ȼ���
(5)Eװ�ó����������⣬�������л����E�з�����������ѷ���Ϊ (����뷽������)��
(6)��װ�û���ȱ�ݣ�ԭ����û�н���β����������β����Ҫ�ɷ�Ϊ (����)��
A.CH4��B��CH3Cl��C��CH2C12��D��CHCl3��E��CCl4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ij�о���ѧϰС���һ�������ǡ�NO2�ܷ�֧��ľ����ȼ�գ�������ʵ������û���ֳɵ�NO2���壬��С���ͬѧ�������������������N2O4�Ĵ��ڣ�ͼ������̨�ȼг�����������ȥ����
I��ʵ��װ������ͼ��ʾ��5�֣�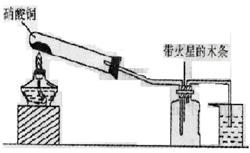
��1������ͭ������ȷֽ�IJ���������ͭ������������������д���÷�Ӧ�Ļ�ѧ����________________��
��2��������ƿ�г�������ɫ����ʱ��ľ����ȼ�ˡ��е�ͬѧ�ó���NO2��֧��ľ����ȼ�ա��Ľ��ۡ�����Ϊ��һ�����Ƿ���ȷ��____________�����ȷ������ȷ������������_______
II��ʵ��װ����ͼ��ʾ����5�֣�
��1��д��Ũ�������ȷֽ�Ļ�ѧ����ʽ��__________________________________����2�֣�
��2��ʵ�鿪ʼ��ľ���ϵĻ�����Ϩ���е�ͬѧ�����NO2����֧�� ľ����ȼ�ա��Ľ��ۡ�����Ϊ��һ�����Ƿ���ȷ��________�����ȷ������ȷ������������ ����3�֣�
III��Ϊ�˸���ֱ��˵����NO2�ܷ�֧��ľ����ȼ�ա���һ���⣬�����������һ����ʵ�鷽��������ʵ��ԭ������Ҫ��������2�֣�_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʯ����Ҫ�ɷ�ΪCaC2������ˮ��Ӧ����C2H2����C2H2�׳Ƶ�ʯ����ijͬѧ������·����ⶨ��ʯ��CaC2�Ĵ���(���ʲ��μӷ�Ӧ)��
ʵ��ԭ����CaC2 +2H2O==Ca(OH)2+C2H2 ��
ͨ���ⶨ���ɵ���������(��״��������ȷ����Ʒ��CaC2�ĺ�����
ʵ�鲽�裺
�ٳ�ȡ��Ʒ1.2g��
�ڰ�1.2g��Ʒ�������������õ����巢��װ�ã���ͼ��ʾ��
������Ʒ�е���ˮ�������ٲ������ݣ�����Ͳ��ˮ��������Ͳ��Һ����360mL��ǡ��ˮ����Һ����ƽ��
����Ϊ��Ӧ������ƿ����24mLҺ�塣
��ش��������⣺
��1������ʵ�����õIJ��������е��ܡ�ˮ�ۡ� �� �� ��
��2����ʵ��ǰ��1000mL��500 mL��250 mL����Ͳ���ã���Ӧѡ�� mL����Ͳ��
��3��������������� mL��Ϊ��֤�������������ȷ�ԣ���ȡ��Ͳ�̶�ʱӦע��������� ��
��4������C2H2ͨ��KMnO4��Һ�У�KMnO4��Һ���Ϻ�ɫ���dz����Ӧ�Ļ�ѧ����ʽΪ��KMnO4+��C2H2����H2SO4������K2SO4����MnSO4����CO2���� ������ʵ�����ɵ�C2H2��ȫ��KMnO4��Һ������������0.1 mol��L-1��KMnO4��Һ mL��
��5��ͨ���������ݼ��㣬�ɵ���Ʒ��CaC2�Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ҫ������NaCl��Һ����ʵ���ҵ�NaCl�����������Na2SO4��NH4HCO3��ijͬѧ����������ͼ���ʵ���ȥ���ʣ��ش��������⣺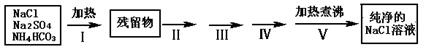
��1������I��ȥ�������ǣ��ѧʽ��_______________��ֱ�Ӽ���Ҫ���ڼ�ǿ����ٽ��м��ȣ������� ��
��2��������ͼ���ʵ����ƣ�����ص�ʵ�������ʵ�������ʵ��Ŀ����д���±��У�
| �������� | ʵ������ | ʵ��Ŀ�� |
| ����II�����������ܽ�õ���Һ�� | | |
| ����III�� | 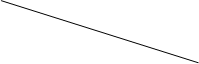 | |
| ����IV�����ˣ�����Һ�� | | |
| ����V������Һ������� |  | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ô����˫��ˮ��Ͽ���������Һ��ϴ�Ӽ���2Na2CO3?3H2O2����������ɱ������ȥ���۵������Ҳ�����ȾˮԴ��
��1��������������ϴ�Ӽ��н��������ӵIJ�����������______________________��
��2������ϴ�Ӽ��е�˫��ˮ���Խ���ˮ�е��軯��ת��Ϊ����ͬʱ����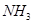 ��д����Ӧ�����ӷ���ʽ____________________________��
��д����Ӧ�����ӷ���ʽ____________________________��
��3���������ϴ�Ӽ���ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ������ӷ���ʽ�ͼ�Ҫ���ֱ�������
__________________________________________________��
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������ϴ�Ӽ��IJ���Ӱ�죬ȡ��ϴ�Ӽ�100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.10 mol?L��1NaOH��Һ��8.0 mol?L��1NaOH��Һ������ʯ��ˮ��0.01 mol?L��1KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������_____�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й�����Ӧ��Ʒ�к��е������������
| A������÷ζ�������ȣ��ʣ�һ�� ������������ |
| B������ˮ�ϰɣ��ȳ�����ζ�������Ҵ� |
| C�������˸��иƣ��������ˣ��Ȳ�ʹ�ˣ�����Ҳֱ�ˡ�����̼��� |
| D����Ҫ��Ƥ���ã������ô����������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com