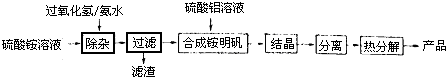
NH
4Al��SO
4��
2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH
4HSO
4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺

��1��NH
4Al��SO
4��
2������ˮ������������
Al3+ˮ�����ɵ�Al��OH��3���������ԣ���Al3++3H2O�TAl��OH��3+3H+��Al��OH��3������������ʹ������Ӷ�����ˮ
Al3+ˮ�����ɵ�Al��OH��3���������ԣ���Al3++3H2O�TAl��OH��3+3H+��Al��OH��3������������ʹ������Ӷ�����ˮ
���ñ�Ҫ�Ļ�ѧ������������˵������
��2����ͬ�����£�0.1mol?L
-1 NH
4Al��SO
4��
2��c��NH
��
��
��
������ڡ������ڡ���С�ڡ���0.1mol?L
-1 NH
4HSO
4��c��NH
����
��3����ͼ1��ʾ��0.1mol?L
-1�������Һ��pH���¶ȱ仯��ͼ��
�������0.1mol?L
-1 NH
4Al��SO
4��
2��pH���¶ȱ仯��������
��
��
����д��ţ�������pH���¶ȱ仯��ԭ����
NH4Al��SO4��2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶�����pH��С
NH4Al��SO4��2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶�����pH��С
��
��20��ʱ��0.1mol?L
-1 NH
4Al��SO
4��
2��2c��SO
��-c��NH
��-3c��Al
3+��=
10-3mol?L-1
10-3mol?L-1
������ֵ����
��4������ʱ����100mL 0.1mol?L
-1 NH
4HSO
4��Һ�еμ�0.1mol?L
-1 NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������
a
a
�㣻��b�㣬��Һ�и�����Ũ���ɴ�С������˳����
c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+��
c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+��
��