
���ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
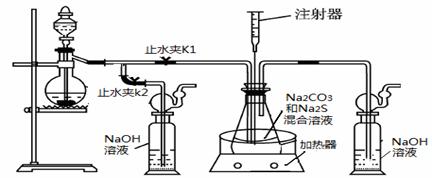 2Na2 S +Na2CO3 + 4SO2= 3Na2S2O3 +CO2������Ҫ��ش����⣺
2Na2 S +Na2CO3 + 4SO2= 3Na2S2O3 +CO2������Ҫ��ش����⣺
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2=2NaI+ Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
��ʵ��1��2��Ŀ����̽����������Ũ�ȶ���������ת���ʵ�Ӱ�죬��a=
������Ҫ̽����ҺpH����Ӧ�¶ȡ������������������ת���ʵ�Ӱ�죬��ʵ��1��2�⣬���ٻ������ �ζԱ�ʵ��
��ʵ���������������ת���ʲ�������������ٵ�Ӱ�졣Ϊʲô��
��________________________________________ ___��
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 0.496v |
| m |
| 0.496v |
| m |
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1.5��1 | 100 | 18 | 80.7% |
| 2 | a | 1.1��1 | 100 | 18 | 94.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��κ�һ�и�����һ�������Ի�ѧ�� ���ͣ�ʵ����
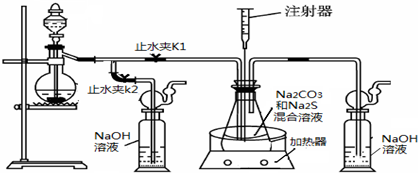
��16�֣����ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
2Na2 S +Na2CO3 + 4SO2  3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2="2NaI+" Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1.5��1 | 100 | 18 | 80.7% |
| 2 | a | 1.1��1 | 100 | 18 | 94.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1.5��1 | 100 | 18 | 80.7% |
| 2 | a | 1.1��1 | 100 | 18 | 94.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
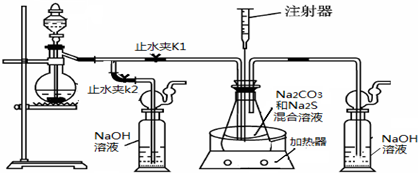
���ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
2Na2 S +Na2CO3 + 4SO2 ![]() 3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
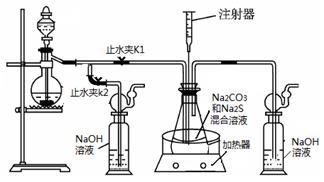
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2=2NaI+ Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com