

 �� �� ��
�� �� �� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʱ�����õ�Br2�������õ�NaOH��H2 |
| B����ȡ������������Һ�е��������Һ����Һ����ɫ |
| C����ȡ������������Һ�е����̪��Һ����Һ���ɫ |
| D����ȡ������������Һ�������Թ��У����������ı������ú��ϲ���Һ�ʳȺ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��101 kPaʱ��1 mol H2ȼ������ˮ�����ų�����������H2��ȼ���� |
| B��0.25molNa2O2�к��е���������ԼΪ0.25��6��02��1023 |
| C����0.2 mol��L-1NaHCO3��Һ�м�������0.1 mol��L-1NaOH��Һ����Ӧ����Һ�У�c(CO32-)> c(HCO3-)> c(OH-)> c(H+) |
| D�����뺣�ڵĸ���բ����װһ��������ͭ��ɷ�ֹբ�ű���ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
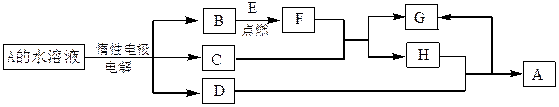 (1)B��ͬ�������������Ϊ_______ __��F�Ļ�ѧʽΪ_____ ___ __��
(1)B��ͬ�������������Ϊ_______ __��F�Ļ�ѧʽΪ_____ ___ __�� ״���µ��������Ϊ_____________��
״���µ��������Ϊ_____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
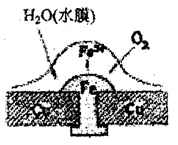
A�������ϵ���Ҫ�缫��ӦʽΪ��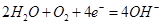 |
| B���˹�����ͭ��δ����ʴ |
| C�����Ӵ�Cu����Fe |
D���˹����л������漰����Ӧ��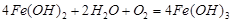 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3��g���ش��������⣺
2NH3��g���ش��������⣺ g���� ��H����483��6kJ��mol
g���� ��H����483��6kJ��mol 2NH3��g���ġ�H��_______________��
2NH3��g���ġ�H��_______________��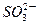 ����c��
����c��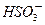 ����c��H2SO3��]��c��
����c��H2SO3��]��c��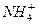 ����c��NH3��H2O��
����c��NH3��H2O��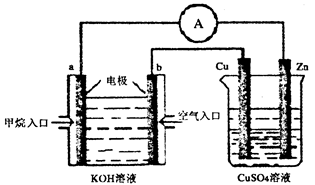
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A�������ĵ缫����ʽΪ��O2 + 2H2O + 4e_ =" 4OH" _ |
| B����ʯī�缫�ij�Mg�缫�����Թ۲쵽�������� |
| C����������ˮ�м�������NaCl(s)���ɽϿ�ؿ������� |
| D���ֱ�������ʯī�缫��������O2��ǰ��������ֵÿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֱ������1nm~100nm ֮������ӳ�Ϊ���� |
| B�������ͨ����Һ������Һ����ɫ��ȥ��˵�������һ������Ư���� |
| C���ں������������п�飬�ɼ�������ĸ�ʴ�ٶ� |
| D�����Ȼ�������Һ�е���⻯����Һ���е⻯���������ɣ�˵���Ȼ������ܽ��С�ڵ⻯�����ܽ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com