
| A��ij��Һ��NaOH��Һ���ȣ�������ʹʪ�����ɫʯ����ֽ�������壬˵��ԭ��Һ�д���笠����� |
| B��ij��Һ�м�����������Һʱ��������ɫ��������ϡ����������ܽ⣬˵��ԭ��Һ�к�Cl- |
| C���ò�˿պȡij��Һ�ھƾ��ƵĻ���������ʱ������ʻ�ɫ��˵��ԭ��Һ��ֻ����Na+�� ������K+�� |
| D��ij��Һ�м���BaCl2��Һʱ��������ɫ��������ϡ����������ܽ⣬˵��ԭ��Һ�д���SO42- |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ù��յķ�����������ͭ��Һ�������������壻 |
| B����ϡ����������BaCl2�� NaCl�� Na2CO3������Һ�� |
| C����BaCl2��Һ��ϡ����������Na2SO4��AgNO3������Һ�� |
| D����CCl4������FeCl3��Һ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������Fe��OH��3�����FeCl3�Ļ���� |
| B���ýᾧ���ᴿNaCl��KNO3�е�KNO3 |
| C�����������Ҵ��ͱ��ӵĻ������ӵķе�182�棩 |
| D���ü��ȷ��������Ȼ�淋Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���� | B��ϡ���� |
| C����������Һ | D���Ȼ�þ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
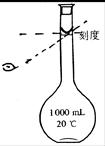
| A����������ƿ��ʢԼ1/2���������ˮ�������뼸�����Ƭ |
| B�����¶ȼ�ˮ����嵽������ƿ�е�����ˮ�� |
| C����ˮӦ���������¿ڽ����Ͽڳ� |
| D���ռ�����Һ��ȡ����������������ϡ���ᣬ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaHCO3��Һ��Na2CO3����CO2 | B�����ۣ����ۣ���NaOH��Һ |
| C��FeCl3��Һ��AlCl3������ˮ | D��NaCl��Һ��Na2CO3�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com