
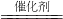 4CO2+2N2���ʴ�Ϊ��4CO+2 NO2
4CO2+2N2���ʴ�Ϊ��4CO+2 NO2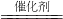 4CO2+2N2��
4CO2+2N2��

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
I����֪��Ӧ��
3I����aq��+S2O82��(aq) ![]() I3��(aq)+2SO42��(aq);
I3��(aq)+2SO42��(aq);
��1��д����Ӧ��ƽ�ⳣ������ʽ
 K= ��
K= ��
��2����ͼ��ʾ��Ӧ�������й����ʵ���������Ӧ
�� ��H 0����>��<��=����
��I������II�������У�ʹ�ô������� �ߡ�
��3����Ӧ�з�����Ӧ��I���뱻������I�������ʵ�����Ϊ ��
��4����Ӧ�����ʿ�����I3�������ĵ�����Һ��Ӧ����ɫ��ʱ��t��������tԽС����Ӧ����Խ��ij̽����ѧϰС����20�����ʵ�飬��¼���������£�
| ʵ���� | �� | �� | �� | �� | �� |
| c(I��)/mol��L��1 | 0.040 | 0.080 | 0.080 | 0.160 | 0.160 |
| c(S2O82��/mol��L��1) | 0.040 | 0.040 | 0.080 | 0.080 | 0.040 |
| t/s | 88 | 44 | 22 | 11 | t1 |
��ʵ���Ŀ����
��ɫʱ��t1= s
�����������ݣ��ó��Ľ�����
II����������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
����������SO2����������SO3��2SO2(g)+O2(g) ![]() 2SO3(g)
2SO3(g)
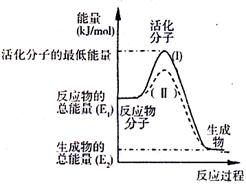
ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ��
 ����ͼʾ�ش��������⡣
����ͼʾ�ش��������⡣
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A�� K��B��
���>������<������=������
����֪������Ӧ���Ƿ��ȷ�Ӧ�����÷�Ӧ����ƽ��״̬ʱ��
���������������£�����ѡ�������������SO2ƽ��ת
���ʵ��� ������ĸ����
A�������¶� B�������¶� C������ѹǿ D����Сѹǿ
E��������� F���Ƴ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com