��ͭ�����ڱ�¶�ڳ�ʪ�����У����ı����������С�ͭ�̡�����ʽ̼��ͭ�����������ֽ⣬Ϊ�˴�ͭ�����Ƶ�ͭ�����ⶨͭ���ڻ�����е�������������ͭ����Ʒ�����Թ��У���ͨ��Ĵ��������ڼ��������·�����Ӧ��ʵ��װ����ͼ1����ش������й����⣮
��1��A��Ӧѡ��ͼ2װ���еģ���д��ţ�
��
��
������̨��ʡȥ����ѡ�õ������Ǣ�
��Ӧ��ˮ���ɣ��Թܿ�Ӧ�Ե����Թܵײ�����ֹ����ˮ�������Թܵײ���ʹ�Թ�ը��
��Ӧ��ˮ���ɣ��Թܿ�Ӧ�Ե����Թܵײ�����ֹ����ˮ�������Թܵײ���ʹ�Թ�ը��
����ͨ�������ĵ�����Ӧ���뵽�Թܵײ��������ڽ��Թ��еĿ����ž���
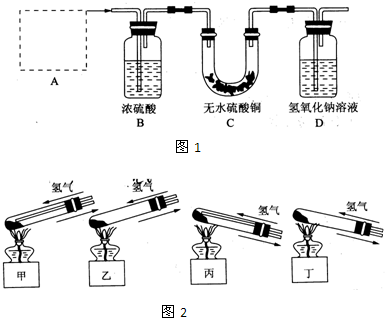
��2����Ӧ��ʼǰ��������ʵ��װ�ñ�����еIJ����dz�����Ʒ���������װ�õ������Ժ�
����װ��D��������������Һ��������
����װ��D��������������Һ��������
����Ӧ����ʱ��Ӧ��
ͨ����
ͨ����
��
����
����
��ʵ������У��۲쵽�Թ��й�����ɫ�仯Ϊ
��������ɫ���ɫ������ɹ����ĺ�ɫ
��������ɫ���ɫ������ɹ����ĺ�ɫ
��
��3��Ҫ�ⶨͭ���ڻ�����е���������������Ҫ֪����Ʒ�������ⶨ��ʵ��������ʵ��ǰ��װ��D������
��4��Ϊʹʵ���ȷ���ɲ�ȡ�Ĵ�ʩ��
��Dװ�ú��һ��ʢ�м�ʯ�ҵ����θ����
��Dװ�ú��һ��ʢ�м�ʯ�ҵ����θ����
��
��10��ʱ����NaHCO
3������Һ����ø���Һ��pH�������±仯
| �¶ȣ��棩 |
10 |
20 |
30 |
������к���ȴ��50�� |
| pH |
8.3 |
8.4 |
8.5 |
8.8 |
��ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO
3-��ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ
HCO3-+H2O?H2CO3+OH-
HCO3-+H2O?H2CO3+OH-
����ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO
3���ȷֽ⣬������Na
2CO
3�����ƶ�Na
2CO
3��ˮ��̶�
����
����
�����С����С�ڡ���NaHCO
3����ͬѧ��Ϊ�ס��ҵ��ж϶�����֣�����Ϊ��
��1��ֻҪ�ڼ�����к����Һ�м����������Լ�X����������������
��
��
����ס����ҡ����ж���ȷ���Լ�X��
B
B
������ţ���
A��Ba��OH��
2��Һ B��BaC1
2��Һ C��NaOH��Һ D�������ʯ��ˮ
��2�������Ⱥ����Һ��ȴ��10�棬����Һ��pH����8.3����
��
��
����ס����ҡ����ж���ȷ��
��3���������ϣ�����NaHCO
3�ķֽ��¶�Ϊ150�棬������
��
��
����ס����ҡ����ж��Ǵ���ģ�������
��ѹ�¼���NaHCO3��ˮ��Һ����Һ���¶ȴﲻ��150��
��ѹ�¼���NaHCO3��ˮ��Һ����Һ���¶ȴﲻ��150��
��




