����������Һ��Ƚ�С��25��ʱ��ÿ100��ˮ���ܽ�0.836�ˣ�
��1��25��ʱ�����ձ��з���6.24�����������壬��200��ˮ��������ܽ�����ñ�����Һ�����Ϊ200������������Һ��Ag+��Ħ��Ũ�ȣ�
��2�����������ձ��м���50����0.0268Ħ/��BaCl2��Һ����ֽ��裬��Һ��Ag+��Ħ��Ũ���Ƕ��٣�
��3���ڣ�1�����ձ�������������0.0268Ħ/��BaCl2��Һ������ʹԭ��Һ��Ag+Ũ�Ƚ�����0.0200Ħ/����
�⣺��1��25��ʱ��ÿ100��ˮ���ܽ�0.836������������200��ˮ�м���6.24�����������壬�ܽ��������������Ϊ2��0.836=1.672g����Ϊ������Һ��
��Һ��n��Ag
2SO
4��=

=0.00268mol��c��Ag
+��=

=0.0536mol/L
����Һ��Ag
+��Ħ��Ũ��Ϊ0.0536mol/L��
��2����Ag
2SO
4�������������Һ��ΪAg
2SO
4������Һ��
��c��Ag
+��=0.0536��Ħ/����
����Һ��Ag
+��Ħ��Ũ��Ϊ0.0536mol/L��
��3��������BaCl
2��Һ�����ΪV������

��mol/L��
V=0.489L
�������0.489��0.0268Ħ/��BaCl
2��Һ������ʹԭ��Һ��Ag
+Ũ�Ƚ�����0.0200Ħ/����
��������1���ж���200��ˮ�м���6.24�����������壬��Һ���ͣ������ܽ�Ƚ��м��㣮
��2��Ag
2SO
4������Һ��Ũ����BaCl
2��Һ��Ũ����ȣ��������Ϊ4��1������μӷ�Ӧ��Ag
2SO
4��������Ϊԭ��Һ��Ag
2SO
4������
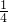
������ԭ�����ˮ��Ag
2SO
4����������Լ�Ag
2SO
4���ܽ�ȿ��жϣ���ʹBaCl
2��Ӧ�����ж����Ag
2SO
4���壮��������Һ��ΪAg
2SO
4������Һ����[Ag
+]���䣻
��3�����ݷ�Ӧ����ʽAg
++Cl
-�TAgCl�����м��㣮
���������⿼�鱥����Һ�ļ�������ʵ���Ũ�ȵļ��㣬�ж����ʷ�Ӧ�ij̶��ǽ����Ĺؼ��������ѶȽϴ�Ϊ1993��߿��⣮

 =0.00268mol��c��Ag+��=
=0.00268mol��c��Ag+��= =0.0536mol/L
=0.0536mol/L ��mol/L��
��mol/L��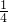 ������ԭ�����ˮ��Ag2SO4����������Լ�Ag2SO4���ܽ�ȿ��жϣ���ʹBaCl2��Ӧ�����ж����Ag2SO4���壮��������Һ��ΪAg2SO4������Һ����[Ag+]���䣻
������ԭ�����ˮ��Ag2SO4����������Լ�Ag2SO4���ܽ�ȿ��жϣ���ʹBaCl2��Ӧ�����ж����Ag2SO4���壮��������Һ��ΪAg2SO4������Һ����[Ag+]���䣻

