

ͼ3-4
����ȥB����֪����ǿ�Ȣ�A������C������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪ����sAB������A
��֪A��B��C�ֱ�ѡ��������Һ��
��0.1 mol��L-1���� ��0.1 mol��L-1���� ��0.1 mol��L-1NaCl��Һ ��0.1 mol��L-1���� ��0.1 mol��L-1 NaOH��Һ ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7
�ش��������⣺
(1)ָ��A��B��C��(�������)ʲô��Һ?
A____________��B____________��C____________��
(2)����C��Һ�е����̪�Լ��ʺ�ɫ����C��____________����A��B��C�ֱ��Ե��������������ϣ������������ϵĻ��Һ�У�ˮ�ĵ���̶����? ____________��(ѡ�A����B����C��)
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
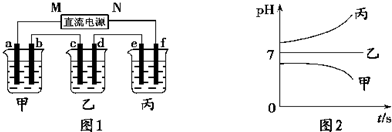
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
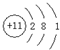

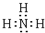
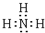
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
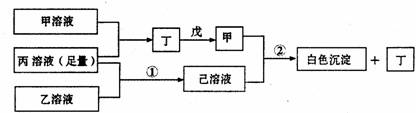
��A��B��C���ֶ�����Ԫ�أ����ǵ�ԭ��������������BԪ��ԭ��������������CԪ��ԭ��������������һ�룬AԪ��ԭ��������������BԪ��ԭ��������������һ�����ס��ҡ���������A��B��C��Ԫ����ۺ���������Σ��ס�����ҺpH>7������ҺpH<7����Ϊ���壬��Ϊ����ɫ���塣�ס��ҡ����������졢����������֮������Ӧ��ϵ����ͼ�����ֲ�������ȥ����
��1��A��Ԫ������Ϊ ��CԪ�صļ����ӽṹʾ��ͼΪ ��
��2��д����Ӧ�ٵ����ӷ���ʽ ��
��3������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ ��
��4��������ͭ�缫�������Һ�н��е�⣬��ʼʱ���������ӷ���ʽΪ ��
��5��д����Ӧ�ڵĻ�ѧ����ʽ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
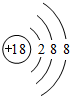
��A��B��C���������κ�һ������D��A��B�еĽ���Ԫ��Ϊͬ�壬A��D��ˮ��Һ��Ͽ����ɻ�ɫ������B��D��ˮ��Һ��Ͽ����ɰ�ɫ������C��D��ˮ��Һ��Ͽ����ɺ�ɫ��������C����Һ�м���ǿ������������ɫ��״�������ó������ڹ�����ǿ���С��ò���������ͭ�����������D����Һ����������������ɫ������
��д��A��B��C��D�ķ���ʽ��(2��)
��д��A��B��C��D����Һ��Ӧ�����ӷ���ʽ��(3��)
��д��C��ǿ����Һ��Ӧ�����ӷ���ʽ��(2��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com