
 ��0.8mol I2��g����1.2mol H2��g������ij1L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g�����ﵽƽ�⣮HI�����������ʱ��ı仯�������ʾ��
��0.8mol I2��g����1.2mol H2��g������ij1L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g�����ﵽƽ�⣮HI�����������ʱ��ı仯�������ʾ��| HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min |
| ����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
| ����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
���� ��1���ɱ������ݿ�֪��5minʱ����ƽ��״̬����I2����Ũ��Ϊxmol/L����
I2��g��+H2��g��?2HI��g��
��ʼŨ�ȣ�mol/L����0.8 1.2 0
ת��Ũ�ȣ�mol/L����x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x
����HI����������з��̼���x���ٸ���K=$\frac{{c}^{2}��HI��}{c��{I}_{2}����c��{H}_{2}��}$���㣻
��2������v=$\frac{��c}{��t}$���㣻
��3����ͬʱ����HI�����������С��˵����Ӧ���ʼ�����ƽ��ʱHI���������������Iʱ���ʸı�����ƽ�������ƶ�������ѹǿ��������Ӱ��ƽ���ƶ��������ǽ����¶ȣ�
��4�������¶�ƽ��ʱ�����ƶ���˵������ӦΪ���ȷ�Ӧ��
��5��������I�´ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�룬ѹǿ����ƽ�ⲻ�ƶ���HI��Ũ�ȱ�Ϊԭƽ���2����
��� �⣺��1����I2����Ũ��Ϊxmol/L����
I2��g��+H2��g��?2HI��g��
��ʼŨ�ȣ�mol/L����0.8 1.2 0
ת��Ũ�ȣ�mol/L����x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%����$\frac{2x}{2}$=60%����x=0.6��ƽ�ⳣ��K=$\frac{{c}^{2}��HI��}{c��{I}_{2}����c��{H}_{2}��}$=$\frac{1��{2}^{2}}{0.2��0.6}$=12��
�ʴ�Ϊ��12��
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ$\frac{0.6mol/L}{5min}$=0.12 mol/��L•min����
�ʴ�Ϊ��0.12 mol/��L•min����
��3����ͬʱ����HI�����������С��˵����Ӧ���ʼ�����ƽ��ʱHI���������������Iʱ���ʸı�����ƽ�������ƶ�������ѹǿ��������Ӱ��ƽ���ƶ��������ǽ����¶ȣ�
�ʴ�Ϊ�������¶ȣ�
��4�������¶�ƽ��ʱ�����ƶ���˵������ӦΪ���ȷ�Ӧ������H��0��
�ʴ�Ϊ������
��5��������I�´ﵽƽ���HI��Ũ��Ϊ1.2mol/L����7minʱ���������ѹ��Ϊԭ����һ�룬ѹǿ����ƽ�ⲻ�ƶ���HI��Ũ�ȱ�Ϊԭƽ���2������HIŨ�ȱ�Ϊ2.4mol/L��c��HI����ʱ��仯������Ϊ��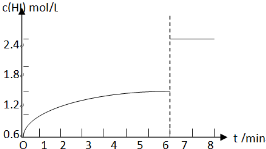 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���Ӧ���ʼ��㡢ƽ�ⳣ���ȣ�ע��ӣ�3���з�Ӧ������ƽ���ƶ��жϸı�����������ȷ����4�����ʱ䣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | ̼��ƺʹ��ᷴӦ��CaCO3+2CH3COOH�TCa2++2CH3COO-+CO2��+H2O | |
| C�� | п����������Һ��Ӧ��Zn+Ag+�TZn2++Ag | |
| D�� | ����ͭ��ϡ���ᷴӦ��CuO+H+�TCu2++H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Ԫ�ؿ����γɶ����⻯���NH3��N2H4�ȣ�
��Ԫ�ؿ����γɶ����⻯���NH3��N2H4�ȣ�| T/�� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �´ﵽƽ��ʱH2��ת����Ϊ33.3%�����¶��µ�ƽ�ⳣ��K��ֵΪ$\frac{100}{27}$���������¶ȣ�Kֵ��С���������С�����䡱����
�´ﵽƽ��ʱH2��ת����Ϊ33.3%�����¶��µ�ƽ�ⳣ��K��ֵΪ$\frac{100}{27}$���������¶ȣ�Kֵ��С���������С�����䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 L�����й��ۼ���ĿΪ19NA | |
| B�� | 12.4g�������к��е�P-P������0.1NA | |
| C�� | 2mol SO2��1mol O2�����V2O5���ڵ������£��ܱ������м��ȷ�Ӧ�����������ʵķ���������2NA | |
| D�� | ��NO2��N2O4���ӹ�NA����������״���£������Ϊ22.4 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ҵ������������ʱ����ͬʱ����һ�ֳ�������Ҫ������������Ư���������ƣ�NaClO2���������������£�
��ҵ������������ʱ����ͬʱ����һ�ֳ�������Ҫ������������Ư���������ƣ�NaClO2���������������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 71g | B�� | 71 | C�� | 71g/mol | D�� | 142g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com