
 ��ͼ��ʵ��װ�ÿ�����ʵ��������ȡ��Ȳ����ش��������⣺
��ͼ��ʵ��װ�ÿ�����ʵ��������ȡ��Ȳ����ش��������⣺


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
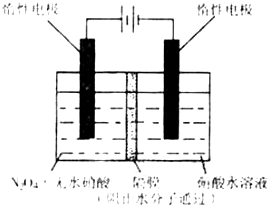 ��2008?���죩N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��2008?���죩N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
| t/s | 0 | 500 | 1000 |
| e��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
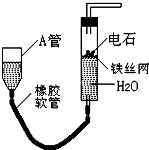 ��ͼ��ʵ��װ�ÿ�����ʵ��������ȡ��Ȳ����ش��������⣺
��ͼ��ʵ��װ�ÿ�����ʵ��������ȡ��Ȳ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�������������������ѧ2010��2011ѧ��߶�3���¿���ѧ���� ���ͣ�058
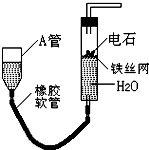
����ͼ��ʵ��װ�ÿ�����ʵ��������ȡ��Ȳ��
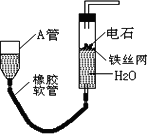
��ش��������⣺
(1)ͼ�У�A�ܵ�������________����ȡ��Ȳ�Ļ�ѧ����ʽΪ________��
(2)���Ƶõ���Ȳͨ������KMnO4��Һ�пɹ۲쵽��������________������������Ȳ������________��Ӧ��
(3)���Ƶõ���Ȳͨ��������Ȼ�̼��Һ�пɹ۲쵽��������________������������Ȳ������________��Ӧ����Ӧ��ѧ����ʽΪ________
(4)Ϊ�˰�ȫ����Ȳ�����ڵ�ȼǰӦ________����Ȳȼ��ʱ��������________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0119 ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com